Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
3-Hydroxycinnamic acid (M2MDB001580)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-07-30 14:54:45 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-09-13 12:56:14 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 3-Hydroxycinnamic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 3-hydroxycinnamic acid, also known as m-coumaric acid, is an aromatic acid. Research has shown that E. coli K-12 can grow with 3-hydroxycinnamic acid as the sole carbon source. (EcoCyc, PMID 6345502) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
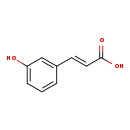
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H8O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 164.158 Monoisotopic: 164.047344122 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | KKSDGJDHHZEWEP-SNAWJCMRSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H8O3/c10-8-3-1-2-7(6-8)4-5-9(11)12/h1-6,10H,(H,11,12)/b5-4+ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 588-30-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2E)-3-(3-hydroxyphenyl)prop-2-enoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | m-coumaric acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC(=O)\C=C\C1=CC=CC(O)=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as hydroxycinnamic acids. Hydroxycinnamic acids are compounds containing an cinnamic acid where the benzene ring is hydroxylated. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Phenylpropanoids and polyketides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Cinnamic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Hydroxycinnamic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Hydroxycinnamic acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic homomonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 192-194 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 3-Hydroxycinnamic acid + Hydrogen ion + NADH + Oxygen > Trans-2,3-Dihydroxycinnamate + Water + NAD 3-Hydroxycinnamic acid + Oxygen + NADH + Hydrogen ion <> Trans-2,3-Dihydroxycinnamate + Water + NAD 3-Hydroxycinnamic acid + NADH + Oxygen > Trans-2,3-Dihydroxycinnamate + Water + NAD 3-(3-Hydroxyphenyl)propanoic acid + NADH + Hydrogen ion + Oxygen + 3-Hydroxycinnamic acid <> 3-(2,3-Dihydroxyphenyl)propionic acid + Water + NAD + Trans-2,3-Dihydroxycinnamate 3-Hydroxycinnamic acid + Hydrogen ion + NADH + Oxygen > NAD + Water + 2-Hydroxy-3-(4-hydroxyphenyl)propenoic acid + 2-Hydroxy-3-(4-hydroxyphenyl)propenoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in 3-(3-hydroxyphenyl)propionate hydroxylase activity
- Specific function:
- Catalyzes the insertion of one atom of molecular oxygen into position 2 of the phenyl ring of 3-(3- hydroxyphenyl)propionate (3-HPP) and hydroxycinnamic acid (3HCI)
- Gene Name:
- mhpA
- Uniprot ID:
- P77397
- Molecular weight:
- 62185
Reactions
| 3-(3-hydroxyphenyl)propanoate + NADH + O(2) = 3-(2,3-dihydroxyphenyl)propanoate + H(2)O + NAD(+). |
| (2E)-3-(3-hydroxyphenyl)prop-2-enoate + NADH + O(2) = (2E)-3-(2,3-dihydroxyphenyl)prop-2-enoate + H(2)O + NAD(+). |
Transporters
- General function:
- Involved in transmembrane transport
- Specific function:
- Could be a transporter for 3-phenylpropionate (hydrocinnamic acid)
- Gene Name:
- mhpT
- Uniprot ID:
- P77589
- Molecular weight:
- 41550
- General function:
- Involved in transporter activity
- Specific function:
- Non-specific porin
- Gene Name:
- ompN
- Uniprot ID:
- P77747
- Molecular weight:
- 41220
- General function:
- Involved in transporter activity
- Specific function:
- Uptake of inorganic phosphate, phosphorylated compounds, and some other negatively charged solutes
- Gene Name:
- phoE
- Uniprot ID:
- P02932
- Molecular weight:
- 38922
- General function:
- Involved in transporter activity
- Specific function:
- OmpF is a porin that forms passive diffusion pores which allow small molecular weight hydrophilic materials across the outer membrane. It is also a receptor for the bacteriophage T2
- Gene Name:
- ompF
- Uniprot ID:
- P02931
- Molecular weight:
- 39333
- General function:
- Involved in transporter activity
- Specific function:
- Forms passive diffusion pores which allow small molecular weight hydrophilic materials across the outer membrane
- Gene Name:
- ompC
- Uniprot ID:
- P06996
- Molecular weight:
- 40368