| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-07-30 14:54:39 -0600 |
|---|
| Update Date | 2015-06-03 17:20:40 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
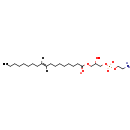
| Name: | 1-Acyl-sn-glycero-3-phosphoethanolamine (N-C18:1) |
|---|
| Description | 1-acyl-sn-glycero-3-phosphoethanolamine (n-c18:1) belongs to the class of Lysophosphatidylglycerols. These are glycerophosphoglycerols (molecules containing a glycerol moiety attached to the phosphate group linked to a glycerol) in which only one fatty acid is bonded to the 1-glycerol moiety (through an ester linkage). (inferred from compound structure) |
|---|
| Structure | |
|---|
| Synonyms: | Not Available |
|---|
| Chemical Formula: | C23H43NO7P |
|---|
| Weight: | Average: 476.5638
Monoisotopic: 476.277714247 |
|---|
| InChI Key: | PRJOHPHYHOVCBM-MDZDMXLPSA-M |
|---|
| InChI: | InChI=1S/C23H44NO7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-23(26)29-20-22(25)21-31-32(27,28)30-19-18-24/h9-10,22,25H,2-8,11-21H2,1H3,(H,27,28)/q+1/p-1/b10-9+ |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | {2-[(2-hydroxy-3-{[(9E)-1-(λ³-oxidanyliumylidene)octadec-9-en-1-yl]oxy}propyl phosphonato)oxy]ethyl}azanylidene |
|---|
| Traditional IUPAC Name: | {2-[(2-hydroxy-3-{[(9E)-1-(λ³-oxidanyliumylidene)octadec-9-en-1-yl]oxy}propyl phosphonato)oxy]ethyl}azanylidene |
|---|
| SMILES: | [H]\C(CCCCCCCC)=C(\[H])CCCCCCCC(=[O+])OCC(O)COP([O-])(=O)OCC[N] |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glycerophospholipids. These are derivatives of glycerophosphoric acid that contains at least one O-acyl, or O-alkyl, or O-(1-alkenyl) group attached to the glycerol residue. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Glycerophospholipids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glycerophospholipid
- Dialkyl phosphate
- Fatty acid ester
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Secondary alcohol
- Carboxylic acid ester
- Organic nitrene
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic zwitterion
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | CPD0-2150 | | EcoCyc ID | CPD0-2150 |
|
|---|