| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:51:42 -0600 |
|---|
| Update Date | 2015-06-03 17:20:20 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
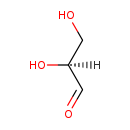
| Name: | L-Glyceraldehyde |
|---|
| Description | Glyceraldehyde is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet colorless crystalline solid that is an intermediate compound in carbohydrate metabolism. The word comes from combining glycerine and aldehyde, as glyceraldehyde is merely glycerine with one hydroxide changed to an aldehyde. |
|---|
| Structure | |
|---|
| Synonyms: | - (+-)-glyceraldehyde
- (+/-)-2,3-dihydroxy-Propanal
- (+/-)-glyceraldehyde
- (2R)-2,3-Dihydroxypropanal
- (S)-2,3-dihydroxypropanal
- .alpha.,.beta.-dihydroxypropionaldehyde
- 2,3-Dihydroxy-(.+/-.)-Propanal
- 2,3-Dihydroxy-(S)-Propanal
- 2,3-Dihydroxy-Propanal
- 2,3-Dihydroxy-Propionaldehyde
- 2,3-Dihydroxypropanal
- 2,3-Dihydroxypropionaldehyde
- 3,6-Dihydroxy-1,4-dioxane-2,5-dimethanol
- a,b-Dihydroxypropionaldehyde
- Aldotriose
- Alpha,beta-Dihydroxypropionaldehyde
- D-(+)-Glyceraldehyde
- D-2,3-Dihydroxypropanal
- D-2,3-Dihydroxypropionaldehyde
- D-Aldotriose
- D-Glyceraldehyde
- D-Glycerose
- Delta-(+)-Glyceraldehyde
- Delta-2,3-Dihydroxypropanal
- Delta-2,3-Dihydroxypropionaldehyde
- Delta-Aldotriose
- Delta-Glyceraldehyde
- Delta-Glycerose
- Dihydroxypropionaldehyde
- DL-GLYC
- DL-glyceraldehyde
- DL-glyceraldehyde dimer
- DL-glyceraldehyde, dimer
- DLG
- G4802_ALDRICH
- G5001_SIGMA
- Gliceraldehido
- Glycerald
- Glyceraldehyd
- Glyceraldehyde
- Glyceric aldehyde
- Glycerinaldehyd
- Glycerinaldehyde
- Glycerinformal
- Glycerose
- Glyzerinaldehyd
- GOL
- L-(-)-Glyceraldehyde
- L-2,3-Dihydroxypropanal
- L-2,3-Dihydroxypropionaldehyde
- L-Aldotriose
- L-Glycerose
- WLN: VHYQ1Q
- α,β-Dihydroxypropionaldehyde
- δ-(+)-Glyceraldehyde
- δ-2,3-Dihydroxypropanal
- δ-2,3-Dihydroxypropionaldehyde
- δ-Aldotriose
- δ-Glyceraldehyde
- δ-Glycerose
|
|---|
| Chemical Formula: | C3H6O3 |
|---|
| Weight: | Average: 90.0779
Monoisotopic: 90.031694058 |
|---|
| InChI Key: | MNQZXJOMYWMBOU-GSVOUGTGSA-N |
|---|
| InChI: | InChI=1S/C3H6O3/c4-1-3(6)2-5/h1,3,5-6H,2H2/t3-/m1/s1 |
|---|
| CAS number: | 497-09-6 |
|---|
| IUPAC Name: | (2S)-2,3-dihydroxypropanal |
|---|
| Traditional IUPAC Name: | L-(-)-glyceraldehyde |
|---|
| SMILES: | [H][C@](O)(CO)C=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monosaccharides. Monosaccharides are compounds containing one carbohydrate unit not glycosidically linked to another such unit, and no set of two or more glycosidically linked carbohydrate units. Monosaccharides have the general formula CnH2nOn. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Monosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monosaccharide
- Alpha-hydroxyaldehyde
- Secondary alcohol
- 1,2-diol
- Organic oxide
- Hydrocarbon derivative
- Short-chain aldehyde
- Primary alcohol
- Carbonyl group
- Aldehyde
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 0 |
|---|
| Melting point: | 145 °C |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|