| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:50:22 -0600 |
|---|
| Update Date | 2015-06-03 17:20:17 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
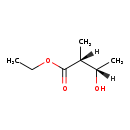
| Name: | Ethyl-(2R)-methyl-(3S)-hydroxybutanoate |
|---|
| Description | Ethyl-(2r)-methyl-(3s)-hydroxybutanoate belongs to the class of Fatty Acid Esters. These are carboxylic ester derivatives of a fatty acid. (inferred from compound structure) |
|---|
| Structure | |
|---|
| Synonyms: | - 3-Hydroxy-2-methyl-ethyl ester(2R,3S)-Butanoate
- 3-Hydroxy-2-methyl-ethyl ester(2R,3S)-Butanoic acid
- Ethyl (2R,3S)-3-hydroxy-2-methylbutanoate
- Ethyl (2R,3S)-3-hydroxy-2-methylbutanoic acid
- Ethyl-(2R)-methyl-(3S)-hydroxybutanoic acid
- ETHYL-2R-METHYL-3S-HYDROXYBUTANOATE
- ETHYL-2R-methyl-3S-hydroxybutanoic acid
|
|---|
| Chemical Formula: | C7H14O3 |
|---|
| Weight: | Average: 146.1843
Monoisotopic: 146.094294314 |
|---|
| InChI Key: | BZFWGBFTIQSEBN-RITPCOANSA-N |
|---|
| InChI: | InChI=1S/C7H14O3/c1-4-10-7(9)5(2)6(3)8/h5-6,8H,4H2,1-3H3/t5-,6+/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | ethyl (2R,3S)-3-hydroxy-2-methylbutanoate |
|---|
| Traditional IUPAC Name: | ethyl (2R,3S)-3-hydroxy-2-methylbutanoate |
|---|
| SMILES: | [H][C@@](C)(O)[C@@]([H])(C)C(=O)OCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acid ester
- Beta-hydroxy acid
- Fatty acyl
- Secondary alcohol
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|