| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:48:38 -0600 |
|---|
| Update Date | 2015-06-03 17:20:08 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
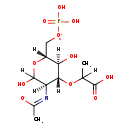
| Name: | N-Acetylmuramate 6-phosphate |
|---|
| Description | MurNAC-6-P is a member of the chemical class known as Sugar Acids and Derivatives. These are compounds containing a saccharide unit which bears a carboxylic acid group. It is a substrate for N-acetylmuramate-6-phosphate etherase. This enzyme catalyzes the cleavage of the D-lactyl ether of MurNAC-6-P to produce GlcNAC-6-P and D lactate. It is required for growth on MurNAC and plays a role in cell wall biogenesis and peptidoglycan recylcing. |
|---|
| Structure | |
|---|
| Synonyms: | - MurNAc-6-P
- N-Acetylmuramate 6-phosphate
- N-Acetylmuramic acid 6-phosphate
- N-Acetylmuramic acid 6-phosphoric acid
|
|---|
| Chemical Formula: | C11H19NO11P |
|---|
| Weight: | Average: 372.2424
Monoisotopic: 372.069571967 |
|---|
| InChI Key: | METUMBUEJVSNMZ-NFSFVEDMSA-M |
|---|
| InChI: | InChI=1S/C11H20NO11P/c1-4(10(15)16)22-9-7(12-5(2)13)11(17)23-6(8(9)14)3-21-24(18,19)20/h4,6-9,11,14,17H,3H2,1-2H3,(H,12,13)(H,15,16)(H2,18,19,20)/q+1/p-1/t4?,6-,7-,8-,9-,11?/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 2-{[(3R,4R,5S,6R)-2,5-dihydroxy-3-[(1-oxidoethylidene)azaniumyl]-6-[(phosphono-λ³-oxidanyliumyl)methyl]oxan-4-yl]oxy}propanoate |
|---|
| Traditional IUPAC Name: | 2-{[(3R,4R,5S,6R)-2,5-dihydroxy-3-[(1-oxidoethylidene)ammonio]-6-[(phosphono-λ³-oxidanyliumyl)methyl]oxan-4-yl]oxy}propanoate |
|---|
| SMILES: | [H]C(C)(O[C@@]1([H])[C@]([H])(O)[C@@]([H])(C[O+]P(O)(O)=O)OC([H])(O)[C@]1([H])[NH+]=C(C)[O-])C([O-])=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha-hexosamines. These are carbohydrate derivatives containing a hexose moiety in which the oxygen atom is replaced by an n-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-acyl-alpha-hexosamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Muramic_acid
- Hexose phosphate
- N-acyl-alpha-hexosamine
- Monosaccharide phosphate
- Monoalkyl phosphate
- Sugar acid
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Monosaccharide
- Secondary alcohol
- Hemiacetal
- Carboxylic acid salt
- Oxacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Monocarboxylic acid or derivatives
- Ether
- Dialkyl ether
- Carboxylic acid
- Carboxylic acid derivative
- Carboximidic acid derivative
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Organic zwitterion
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | 1,6-anhydro-<i>N</i>-acetylmuramic acid recycling | PW002064 |    |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | - 1,6-anhydro-N-acetylmuramic acid recycling PWY0-1261
|
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 47968 | | HMDB ID | Not Available | | Pubchem Compound ID | 25202911 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | CPD0-881 | | EcoCyc ID | CPD0-881 |
|
|---|