| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:45:05 -0600 |
|---|
| Update Date | 2015-06-03 17:20:01 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Menaquinone-1 |
|---|
| Description | Menaquinone-1 is a menaquinone with 1 isoprenyl group. Menaquinones and demethylmenaquinones are isoprenoid quinones of the naphthalene series, and are constituents of bacterial plasma membranes, where they play important roles in electron transfer and oxidative phosphorylation. Menaquinones or Vitamin K2 homologs are characterized by the number of isoprenoid residues in their side chain. Menaquinones are abbreviated MK-n, where n represents the number of isoprenoid side chain residues. For example, menaquinone-4 (abbreviated MK-4), has four isoprene residues in its side chain. The most common length of the side chain in bacteria is 8, although minor amounts of MK-1 through MK-14 have been found |
|---|
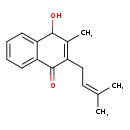
| Structure | |
|---|
| Synonyms: | - 2-Methyl-3-(3-methyl-2-butenyl)naphthoquinone
- Lepachol acetate
- Lepachol acetic acid
- Vitamin MK 1
|
|---|
| Chemical Formula: | C16H18O2 |
|---|
| Weight: | Average: 242.3129
Monoisotopic: 242.13067982 |
|---|
| InChI Key: | PXIPYHVOWDEAMD-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C16H18O2/c1-10(2)8-9-12-11(3)15(17)13-6-4-5-7-14(13)16(12)18/h4-8,15,17H,9H2,1-3H3 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 4-hydroxy-3-methyl-2-(3-methylbut-2-en-1-yl)-1,4-dihydronaphthalen-1-one |
|---|
| Traditional IUPAC Name: | 4-hydroxy-3-methyl-2-(3-methylbut-2-en-1-yl)-4H-naphthalen-1-one |
|---|
| SMILES: | CC(C)=CCC1=C(C)C(O)C2=CC=CC=C2C1=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthalenes. Naphthalenes are compounds containing a naphthalene moiety, which consists of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthalenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthalene
- Monoterpenoid
- Bicyclic monoterpenoid
- Aromatic monoterpenoid
- Aryl ketone
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 25200371 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | CPD0-1367 | | EcoCyc ID | CPD0-1367 |
|
|---|