| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:39:31 -0600 |
|---|
| Update Date | 2015-06-03 17:19:48 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
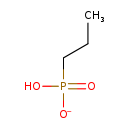
| Name: | Propylphosphonate |
|---|
| Description | Propylphosphonate is a member of the chemical class known as Organic Phosphonic Acids and Derivatives. These are organic compounds containing phosphonic acid or a derivative thereof. . Phosphonates (Pn) are a large class of organophosphorus molecules that have direct carbon-phosphorus (C-P) bonds in place of the carbon-oxygen-phosphorus ester bond. In bacteria two pathways exist for Pn breakdown for use as a P source: the phosphonatase and C-P lyase pathways. These pathways differ both in regard to their substrate specificity and their cleavage mechanism. The phosphonatase pathway acts on the natural Pn alpha-aminoethylphosphonate (AEPn). In a two-step process it leads to cleavage of the C-P bond by a hydrolysis reaction requiring an adjacent carbonyl group. In contrast the C-P lyase pathway has a broad substrate specificity. It leads to cleavage of substituted Pn (such as AEPn) as well as unsubstituted Pn by a mechanism involving redox or radical chemistry. Due to its broad substrate specificity, the C-P lyase pathway is generally thought to be responsible for the breakdown of Pn herbicides (such as glyphosate) by bacteria. |
|---|
| Structure | |
|---|
| Synonyms: | - 1-Propylphosphonate
- 1-Propylphosphonic acid
- PPN
- Propanephosphonate
- Propanephosphonic acid
- Propylphosphonic acid
|
|---|
| Chemical Formula: | C3H8O3P |
|---|
| Weight: | Average: 123.0676
Monoisotopic: 123.021105634 |
|---|
| InChI Key: | NSETWVJZUWGCKE-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C3H9O3P/c1-2-3-7(4,5)6/h2-3H2,1H3,(H2,4,5,6)/p-1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | hydrogen propylphosphonate |
|---|
| Traditional IUPAC Name: | hydrogen propylphosphonate |
|---|
| SMILES: | CCCP(O)([O-])=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organic phosphonic acids. These are organic compounds containing phosphonic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphonic acids and derivatives |
|---|
| Sub Class | Organic phosphonic acids |
|---|
| Direct Parent | Organic phosphonic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organophosphonic acid
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organophosphorus compound
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 25200508 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | CPD0-1076 | | EcoCyc ID | CPD0-1076 |
|
|---|