Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
2'-(5-Triphosphoribosyl)-3'-dephospho-CoA (M2MDB001068)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 14:33:57 -0600 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-06-04 17:06:03 -0600 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2'-(5-Triphosphoribosyl)-3'-dephospho-CoA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 2'-(5-triphosphoribosyl)-3'-dephospho-CoA is a member of the chemical class known as Purine Ribonucleoside Triphosphates. These are purine ribobucleotides with triphosphate group linked to the ribose moiety. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
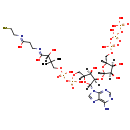
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C26H46N7O26P5S | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 1059.609 Monoisotopic: 1059.090127929 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | NFWZJXFBUKDGOX-JIRKQKIOSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C26H46N7O26P5S/c1-26(2,20(38)23(39)29-4-3-14(34)28-5-6-65)9-53-63(47,48)58-61(43,44)52-8-13-17(36)19(24(54-13)33-11-32-15-21(27)30-10-31-22(15)33)56-25-18(37)16(35)12(55-25)7-51-62(45,46)59-64(49,50)57-60(40,41)42/h10-13,16-20,24-25,35-38,65H,3-9H2,1-2H3,(H,28,34)(H,29,39)(H,43,44)(H,45,46)(H,47,48)(H,49,50)(H2,27,30,31)(H2,40,41,42)/t12-,13-,16-,17-,18-,19-,20+,24-,25+/m1/s1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2R)-4-({[({[(2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-{[(2S,3R,4S,5R)-3,4-dihydroxy-5-({[hydroxy({[hydroxy(phosphonooxy)phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-2-yl]oxy}-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-2-hydroxy-3,3-dimethyl-N-{2-[(2-sulfanylethyl)-C-hydroxycarbonimidoyl]ethyl}butanimidic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (2R)-4-[({[(2R,3R,4R,5R)-5-(6-aminopurin-9-yl)-4-{[(2S,3R,4S,5R)-3,4-dihydroxy-5-[({hydroxy[hydroxy(phosphonooxy)phosphoryl]oxyphosphoryl}oxy)methyl]oxolan-2-yl]oxy}-3-hydroxyoxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]-2-hydroxy-3,3-dimethyl-N-{2-[(2-sulfanylethyl)-C-hydroxycarbonimidoyl]ethyl}butanimidic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H][C@](O)(C(O)=NCCC(O)=NCCS)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@@]1([H])O[C@@]([H])(N2C=NC3=C(N)N=CN=C23)[C@]([H])(O[C@]2([H])O[C@]([H])(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@]([H])(O)[C@@]2([H])O)[C@]1([H])O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organic phosphoric acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Phosphate esters | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Monoalkyl phosphates | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Dephospho-CoA > 2'-(5-Triphosphoribosyl)-3'-dephospho-CoA + Adenine Hydrogen ion + Dephospho-CoA + Adenosine triphosphate > 2'-(5-Triphosphoribosyl)-3'-dephospho-CoA + Adenine 2'-(5-Triphosphoribosyl)-3'-dephospho-CoA + citrate lyase apo-[acyl-carrier-protein] > citrate lyase holo-[acyl-carrier-protein] + Pyrophosphate Adenosine triphosphate + Dephospho-CoA <> 2'-(5-Triphosphoribosyl)-3'-dephospho-CoA + Adenine 2'-(5-Triphosphoribosyl)-3'-dephospho-CoA <> Pyrophosphate 2'-(5-Triphosphoribosyl)-3'-dephospho-CoA + [an apo citrate-lyase acyl-carrier protein] > Pyrophosphate + Hydrogen ion + [a holo citrate lyase acyl-carrier protein] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in prosthetic group biosynthetic process
- Specific function:
- Transfers 2-(5''-triphosphoribosyl)-3'- dephosphocoenzyme-A on a serine residue to the apo-acyl carrier protein (gamma chain) of the citrate lyase to yield holo-acyl carrier protein
- Gene Name:
- citX
- Uniprot ID:
- P0A6G5
- Molecular weight:
- 20270
Reactions
| 2'-(5-triphosphoribosyl)-3'-dephospho-CoA + citrate lyase apo-[acyl-carrier-protein] = citrate lyase holo-[acyl-carrier-protein] + diphosphate. |
- General function:
- Involved in ATP binding
- Specific function:
- Catalyzes the formation of 2-(5''-triphosphoribosyl)-3'- dephosphocoenzyme-A, the precursor of the prosthetic group of the holo-acyl carrier protein (gamma chain) of citrate lyase, from ATP and dephospho-CoA
- Gene Name:
- citG
- Uniprot ID:
- P77231
- Molecular weight:
- 31644
Reactions
| ATP + 3-dephospho-CoA = 2'-(5-triphosphoribosyl)-3'-dephospho-CoA + adenine. |