| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:28:58 -0600 |
|---|
| Update Date | 2015-09-17 16:24:19 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
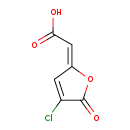
| Name: | Cis-2-Chloro-4-carboxymethylenebut-2-en-1,4-olide |
|---|
| Description | Cis-2-chloro-4-carboxymethylenebut-2-en-1,4-olide is a member of the chemical class known as Aryl Chlorides. These are organic compounds containing the acyl chloride functional group. |
|---|
| Structure | |
|---|
| Synonyms: | - (2E)-[4-chloro-5-oxofuran-2(5H)-ylidene]acetate
- (2E)-[4-chloro-5-oxofuran-2(5H)-ylidene]acetic acid
- (4-chloro-5-oxofuran-2(5H)-ylidene)acetate
- (4-chloro-5-oxofuran-2(5H)-ylidene)acetic acid
- 2-Chloro-4-carboxymethylenebut-2-en-1,4-olide
- Cis-2-chloro-4-carboxymethylenebut-2-en-1,4-olide
- Cis-2-Chlorodienelactone
|
|---|
| Chemical Formula: | C6H3ClO4 |
|---|
| Weight: | Average: 174.539
Monoisotopic: 173.971986291 |
|---|
| InChI Key: | ADSGHWJRPOXXTD-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C6H3ClO4/c7-4-1-3(2-5(8)9)11-6(4)10/h1-2H,(H,8,9) |
|---|
| CAS number: | 115793-61-8 |
|---|
| IUPAC Name: | 2-[(2E)-4-chloro-5-oxo-2,5-dihydrofuran-2-ylidene]acetic acid |
|---|
| Traditional IUPAC Name: | cis-2-chlorodienelactone |
|---|
| SMILES: | OC(=O)C=C1OC(=O)C(Cl)=C1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as butenolides. These are dihydrofurans with a carbonyl group at the C2 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dihydrofurans |
|---|
| Sub Class | Furanones |
|---|
| Direct Parent | Butenolides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-furanone
- Dicarboxylic acid or derivatives
- Enol ester
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Carboxylic acid ester
- Lactone
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Chloroalkene
- Vinyl chloride
- Vinyl halide
- Haloalkene
- Carbonyl group
- Organohalogen compound
- Organochloride
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | - Microbial metabolism in diverse environments ec01120
- gamma-Hexachlorocyclohexane degradation ec00361
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|