Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
5-O-(1-Carboxyvinyl)-3-phosphoshikimate (M2MDB000954)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 14:27:48 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-06-03 17:19:19 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 5-O-(1-Carboxyvinyl)-3-phosphoshikimate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 5-o-(1-carboxyvinyl)-3-phosphoshikimate is a member of the chemical class known as Organophosphate Esters. These are organic compounds containing phosphoric acid ester functional group. The shikimate pathway enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) is the target of the broad spectrum herbicide glyphosate. (PMID 19211556) 5-Enolpyruvylshikimate-3-phosphate (EPSP) synthase (AroA) is a key enzyme in the aromatic amino acid biosynthetic pathway in microorganisms and plants, and is the target of the herbicide glyphosate. (PMID 16469313) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C10H13O10P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 324.178 Monoisotopic: 324.024633148 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | QUTYKIXIUDQOLK-PRJMDXOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C10H13O10P/c1-4(9(12)13)19-6-2-5(10(14)15)3-7(8(6)11)20-21(16,17)18/h3,6-8,11H,1-2H2,(H,12,13)(H,14,15)(H2,16,17,18)/t6-,7-,8+/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 89771-75-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (3R,4S,5R)-5-[(1-carboxyeth-1-en-1-yl)oxy]-4-hydroxy-3-(phosphonooxy)cyclohex-1-ene-1-carboxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (3R,4S,5R)-5-[(1-carboxyeth-1-en-1-yl)oxy]-4-hydroxy-3-(phosphonooxy)cyclohex-1-ene-1-carboxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
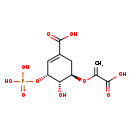
| SMILES: | O[C@H]1[C@@H](CC(=C[C@H]1OP(O)(O)=O)C(O)=O)OC(=C)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organic phosphoric acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Phosphate esters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Monoalkyl phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic homomonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Phosphoenolpyruvic acid + Shikimate 3-phosphate <> 5-O-(1-Carboxyvinyl)-3-phosphoshikimate + Phosphate 5-O-(1-Carboxyvinyl)-3-phosphoshikimate <> Chorismate + Phosphate 5-O-(1-Carboxyvinyl)-3-phosphoshikimate > Phosphate + Chorismate Phosphoenolpyruvic acid + Shikimate 3-phosphate > Inorganic phosphate + 5-O-(1-Carboxyvinyl)-3-phosphoshikimate 5-O-(1-Carboxyvinyl)-3-phosphoshikimate > Chorismate + Inorganic phosphate 5 5-O-(1-Carboxyvinyl)-3-phosphoshikimate <> Chorismate + Phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in transferase activity, transferring alkyl or aryl (other than methyl) groups
- Specific function:
- Phosphoenolpyruvate + 3-phosphoshikimate = phosphate + 5-O-(1-carboxyvinyl)-3-phosphoshikimate
- Gene Name:
- aroA
- Uniprot ID:
- P0A6D3
- Molecular weight:
- 46095
Reactions
| Phosphoenolpyruvate + 3-phosphoshikimate = phosphate + 5-O-(1-carboxyvinyl)-3-phosphoshikimate. |
- General function:
- Involved in chorismate synthase activity
- Specific function:
- 5-O-(1-carboxyvinyl)-3-phosphoshikimate = chorismate + phosphate
- Gene Name:
- aroC
- Uniprot ID:
- P12008
- Molecular weight:
- 39137
Reactions
| 5-O-(1-carboxyvinyl)-3-phosphoshikimate = chorismate + phosphate. |