| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:27:15 -0600 |
|---|
| Update Date | 2015-06-03 17:19:18 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
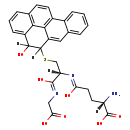
| Name: | 4,5-Dihydro-4-hydroxy-5-S-glutathionyl-benzo[a]pyrene |
|---|
| Description | 4,5-dihydro-4-hydroxy-5-s-glutathionyl-benzo[a]pyrene is a member of the chemical class known as Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. 3-amino-2-propanol is invovled in L-threonine metabolism. D-1-Amino-2-propanol:NAD+ oxidoreductase activity, which catalyzes the second step in a pathway wherein L-threonine is converted to D-1-amino-2-propanol via the intermediate formation of aminoacetone, has been purified 500-fold from Escherichia coli K-12. (PMID 359547) |
|---|
| Structure | |
|---|
| Synonyms: | - (R)-1-aminopropan-2-ol
- 1-Amino-2-propanol
- 3-Amino-2-propanol
- Aminopropanol
- D-1-Amino-2-propanol
- D-1-Aminopropan-2-ol
- DL-1-amino-2-propanol
|
|---|
| Chemical Formula: | C30H29N3O7S |
|---|
| Weight: | Average: 575.632
Monoisotopic: 575.172620987 |
|---|
| InChI Key: | PZXCOGFLSNREMF-SUZCJKPRSA-N |
|---|
| InChI: | InChI=1S/C30H29N3O7S/c31-21(30(39)40)10-11-23(34)33-22(29(38)32-13-24(35)36)14-41-28-20-12-16-4-1-2-6-17(16)18-9-8-15-5-3-7-19(27(28)37)25(15)26(18)20/h1-9,12,21-22,27-28,37H,10-11,13-14,31H2,(H,32,38)(H,33,34)(H,35,36)(H,39,40)/t21-,22-,27?,28?/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-({11-hydroxypentacyclo[10.6.2.0²,⁷.0⁹,¹⁹.0¹⁶,²⁰]icosa-1(19),2,4,6,8,12,14,16(20),17-nonaen-10-yl}sulfanyl)ethyl]-C-hydroxycarbonimidoyl}butanoic acid |
|---|
| Traditional IUPAC Name: | (2S)-2-amino-4-{[(1R)-1-(carboxymethyl-C-hydroxycarbonimidoyl)-2-({11-hydroxypentacyclo[10.6.2.0²,⁷.0⁹,¹⁹.0¹⁶,²⁰]icosa-1(19),2,4,6,8,12,14,16(20),17-nonaen-10-yl}sulfanyl)ethyl]-C-hydroxycarbonimidoyl}butanoic acid |
|---|
| SMILES: | [H][C@](N)(CCC(O)=N[C@@]([H])(CSC1([H])C2=CC3=CC=CC=C3C3=C2C2=C(C=CC=C2C1([H])O)C=C3)C(O)=NCC(O)=O)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Gamma-glutamyl alpha peptide
- Chrysene
- Glutamine or derivatives
- Phenanthrene
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- Cysteine or derivatives
- Alpha-amino acid
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Naphthalene
- L-alpha-amino acid
- Fatty acyl
- N-acyl-amine
- Benzenoid
- Fatty amide
- Dicarboxylic acid or derivatives
- Amino acid
- Amino acid or derivatives
- Secondary alcohol
- Secondary carboxylic acid amide
- Carboxamide group
- Dialkylthioether
- Carboxylic acid
- Sulfenyl compound
- Thioether
- Primary amine
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Amine
- Organic oxide
- Organosulfur compound
- Primary aliphatic amine
- Organooxygen compound
- Organonitrogen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Collection of Reactions without pathways | PW001891 |    | | glutathione metabolism III | PW002018 |    |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-3090070000-318cd5522f8b31ca9e5c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-014i-2090024000-028df145f70e0dd65b29 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("4,5-Dihydro-4-hydroxy-5-S-glutathionyl-benzo[a]pyrene,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_16) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0adj-2011390000-7a7dbf308ba61fa538f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9103720000-1eef252466caae626959 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9142100000-aa9f26588448cdcc954c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0kn9-0133090000-45cda9ad0c81d7f90fd3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f89-0196010000-141a30f61669cde8948a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udl-2942000000-afdbd3b8e287537675d8 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Campbell, R. L., Swain, R. R., Dekker, E. E. (1978). "Purification, separation, and characterization of two molecular forms of D-1-amino-2-propanol:NAD+ oxidoreductase activity from extracts of Escherichia coli K-12." J Biol Chem 253:7282-7288. Pubmed: 359547
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|