Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
1,4-Dihydroxy-2-naphthoyl-CoA (M2MDB000881)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 14:23:53 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-09-17 15:41:52 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 1,4-Dihydroxy-2-naphthoyl-CoA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 1,4-dihydroxy-2-naphthoyl-CoA is a member of the chemical class known as Coenzyme A and Derivatives. These are derivative of vitamin B5 containing a 4'-phosphopantetheine moiety attached to a diphospho-adenosine. 1,4-Dihydroxy-2-naphthoyl-CoA is involved menaquinone biosynthetic pathway. 1,4-dihydroxy-2-naphthoate (DHNA) is a branch point metabolite leading to the biosynthesis of menaquinone (vitamin K2 in bacteria) and begins with the conjugation of DHNA with the CoA moiety via 1,4-Dihydroxy-2-naphthoyl coenzyme A (DHNA-CoA) synthase. (PMID 20643650) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C32H38N7O19P3S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 949.67 Monoisotopic: 949.117798519 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | PYTINLGPKDJURZ-HSJNEKGZSA-J | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C32H42N7O19P3S/c1-32(2,26(44)29(45)35-8-7-21(41)34-9-10-62-31(46)18-11-19(40)16-5-3-4-6-17(16)23(18)42)13-55-61(52,53)58-60(50,51)54-12-20-25(57-59(47,48)49)24(43)30(56-20)39-15-38-22-27(33)36-14-37-28(22)39/h3-6,11,14-15,20,24-26,30,40,42-44H,7-10,12-13H2,1-2H3,(H,34,41)(H,35,45)(H,50,51)(H,52,53)(H2,33,36,37)(H2,47,48,49)/p-4/t20-,24-,25-,26+,30-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2R)-4-({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-N-(2-{[2-(1,4-dihydroxynaphthalene-2-carbonylsulfanyl)ethyl]-C-hydroxycarbonimidoyl}ethyl)-2-hydroxy-3,3-dimethylbutanimidic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (2R)-4-[({[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]-N-(2-{[2-(1,4-dihydroxynaphthalene-2-carbonylsulfanyl)ethyl]-C-hydroxycarbonimidoyl}ethyl)-2-hydroxy-3,3-dimethylbutanimidic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
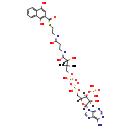
| SMILES: | [H][C@](O)(C([O-])=NCCC([O-])=NCCSC(=O)C1=C([O-])C2=CC=CC=C2C([O-])=C1)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@@]1([H])O[C@@]([H])(N2C=NC3=C(N)N=CN=C23)[C@]([H])(O)[C@]1([H])OP(O)(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Fatty Acyls | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Fatty acyl thioesters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Acyl CoAs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 1,4-Dihydroxy-2-naphthoyl-CoA + Water > Coenzyme A + 1,4-Dihydroxy-2-naphthoic acid + Hydrogen ion Hydrogen ion + 2-Succinylbenzoyl-CoA > 1,4-Dihydroxy-2-naphthoyl-CoA + Water 2-Succinylbenzoyl-CoA <> 1,4-Dihydroxy-2-naphthoyl-CoA + Water 4-(2-Carboxyphenyl)-4-oxobutanoyl-CoA > 1,4-Dihydroxy-2-naphthoyl-CoA + Water 2-Succinylbenzoyl-CoA + Hydrogen ion > Water + 1,4-dihydroxy-2-naphthoyl-CoA + 1,4-Dihydroxy-2-naphthoyl-CoA 1,4-dihydroxy-2-naphthoyl-CoA + Water + 1,4-Dihydroxy-2-naphthoyl-CoA > Coenzyme A + Hydrogen ion + 1,4-dihydroxy-2-naphthoate 1,4-dihydroxy-2-naphthoate + Hydrogen ion + Farnesylfarnesylgeranyl-PP + 1,4-Dihydroxy-2-naphthoyl-CoA > Carbon dioxide + Pyrophosphate + 2-Demethylmenaquinol 8 Octaprenyl diphosphate + 1,4-dihydroxy-2-naphthoate + Hydrogen ion + Octaprenyl diphosphate + 1,4-Dihydroxy-2-naphthoyl-CoA > Carbon dioxide + Pyrophosphate + 2-Demethylmenaquinol 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Converts o-succinylbenzoyl-CoA (OSB-CoA) to 1,4- dihydroxy-2-naphthoyl-CoA (DHNA-CoA)
- Gene Name:
- menB
- Uniprot ID:
- P0ABU0
- Molecular weight:
- 31633
Reactions
| 4-(2-carboxyphenyl)-4-oxobutanoyl-CoA = 1,4-dihydroxy-2-naphthoyl-CoA + H(2)O. |
- General function:
- Involved in transferase activity
- Specific function:
- Conversion of 1,4-dihydroxy-2-naphthoate (DHNA) to dimethylmenaquinone (DMK). Attaches octaprenylpyrophosphate, a membrane-bound 40-carbon side chain to DHNA. The conversion of DHNA to DMK proceeds in three stages:the removal of the carboxyl group of DHNA as CO(2), the attachment of the isoprenoid side chain, and a quinol-to-quinone oxidation, which is thought to be spontaneous
- Gene Name:
- menA
- Uniprot ID:
- P32166
- Molecular weight:
- 33594
Reactions
| An all-trans-polyprenyl diphosphate + 1,4-dihydroxy-2-naphthoate = a demethylmenaquinol + diphosphate + CO(2). |
- General function:
- Involved in hydrolase activity
- Specific function:
- Thioesterase that appears to be involved in phospholipid metabolism. Some specific acyl-ACPs could be physiological substrates. Displays acyl-CoA thioesterase activity on malonyl-CoA in vitro, catalyzing the hydrolysis of the thioester bond
- Gene Name:
- ybgC
- Uniprot ID:
- P0A8Z3
- Molecular weight:
- 15562