| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:23:35 -0600 |
|---|
| Update Date | 2015-06-03 17:19:09 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | 1-Nitro-7-glutathionyl-8-hydroxy-7,8-dihydronaphthalene |
|---|
| Description | 1-nitro-7-glutathionyl-8-hydroxy-7,8-dihydronaphthalene belongs to the class of Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. (inferred from compound structure) |
|---|
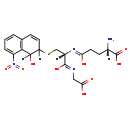
| Structure | |
|---|
| Synonyms: | - (2S)-2-amino-5-(2R)-1-(carboxymethylamino)-3-(1-Hydroxy-8-nitro-1,2-dihydronaphthalen-2-yl)sulfanyl-1-oxopropan-2-ylamino-5-oxopentanoate
- (2S)-2-amino-5-(2R)-1-(carboxymethylamino)-3-(1-hydroxy-8-nitro-1,2-dihydronaphthalen-2-yl)sulfanyl-1-oxopropan-2-ylamino-5-oxopentanoic acid
- (2S)-2-amino-5-(2R)-1-(carboxymethylamino)-3-(1-Hydroxy-8-nitro-1,2-dihydronaphthalen-2-yl)sulphanyl-1-oxopropan-2-ylamino-5-oxopentanoate
- (2S)-2-amino-5-(2R)-1-(carboxymethylamino)-3-(1-Hydroxy-8-nitro-1,2-dihydronaphthalen-2-yl)sulphanyl-1-oxopropan-2-ylamino-5-oxopentanoic acid
- L-g-Glutamyl-S-(1-hydroxy-8-nitro-1,2-dihydro-2-naphthalenyl)-L-cysteinylglycine
- L-gamma-Glutamyl-S-(1-hydroxy-8-nitro-1,2-dihydro-2-naphthalenyl)-L-cysteinylglycine
- L-γ-Glutamyl-S-(1-hydroxy-8-nitro-1,2-dihydro-2-naphthalenyl)-L-cysteinylglycine
|
|---|
| Chemical Formula: | C20H24N4O9S |
|---|
| Weight: | Average: 496.491
Monoisotopic: 496.126399076 |
|---|
| InChI Key: | FCTXJUPCCZHZHU-WXFCVCCESA-N |
|---|
| InChI: | InChI=1S/C20H24N4O9S/c21-11(20(30)31)5-7-15(25)23-12(19(29)22-8-16(26)27)9-34-14-6-4-10-2-1-3-13(24(32)33)17(10)18(14)28/h1-4,6,11-12,14,18,28H,5,7-9,21H2,(H,22,29)(H,23,25)(H,26,27)(H,30,31)/t11-,12-,14?,18?/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-[(1-hydroxy-8-nitro-1,2-dihydronaphthalen-2-yl)sulfanyl]ethyl]-C-hydroxycarbonimidoyl}butanoic acid |
|---|
| Traditional IUPAC Name: | (2S)-2-amino-4-{[(1R)-1-(carboxymethyl-C-hydroxycarbonimidoyl)-2-[(1-hydroxy-8-nitro-1,2-dihydronaphthalen-2-yl)sulfanyl]ethyl]-C-hydroxycarbonimidoyl}butanoic acid |
|---|
| SMILES: | [H][C@](N)(CCC(O)=N[C@@]([H])(CSC1([H])C=CC2=C(C(=CC=C2)N(=O)=O)C1([H])O)C(O)=NCC(O)=O)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthalenes. Naphthalenes are compounds containing a naphthalene moiety, which consists of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthalenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthalene
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic alcohol
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -3 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-2501900000-33399c20b46700295800 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-006x-5901244000-a02271b0549de9b44fcf | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("1-Nitro-7-glutathionyl-8-hydroxy-7,8-dihydronaphthalene,2TBDMS,#4" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000900000-91cfdbb0997afec136d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006t-3000900000-02adc1de44bd9fc5ba98 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9110000000-57cc41b896c948c09835 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000900000-01e07ae0d7e7e473991f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-1110900000-9ca859a4f74cfa0533c5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9830000000-0626d28f448ecdd5bb0e | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|