Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
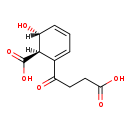
(1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate (M2MDB000851)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 14:22:16 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-09-17 15:41:51 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | (1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylic acid (SHCHC) is the first identified intermediate in the menaquinone biosynthetic pathway. This pathway requires two reactions. They are the decarboxylation of alpha-ketoglutarate by an alpha-ketoglutarate decarboxylase, which results in the formation of succinic semialdehyde-thiamine PPi (TPP) anion, and the addition of the succinic semialdehyde-TPP anion to isochorismate carried out by the enzyme SHCHC synthase. In E. coli, addition of succinic semialdehyde-TPP anion (from 2-oxoglutarate) to isochorismate results in the formation of 2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate (SEPHCHC). In the subsequent reaction, the pyruvoyl group is eliminated, resulting in the prearomatic compound (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate (SHCHC). This is then aromatized to the benzenoid aromatic compound o-succinylbenzoate (OSB) and used as the framework for the construction of the rest of the menaquinone molecule. In this pathway, prenylation in the last step leads to the formation of 1,4-dihydroxy-2-naphtoic acid (DHNA). Subsequently OSB and DHNA formed in this pathway are incorporated into the naphthoquinone ring of menaquinone. SEPHCHC is an unstable compound and in mildly basic solutions, spontaneously undergoes a 2,5-elimination reaction resulting in the formation SHCHC and pyruvate. But the in vivo conversion of SEPHCHC to SHCHC is carried out by SHCHC synthase MenH. Dehydration from SHCHC by the enzyme OSB synthase (MenC) leads to the formation of the benzenoid aromatic compound OSB. The conversion of the benzenoid aromatic compound OSB to the naphthalenoid aromatic compound DHNA is catalyzed by the enzyme OSB-CoA synthetase (MenE). The process has been shown to have an absolute requirement for ATP and CoA. OSB-CoA is suggested as an intermediate. During the formation of OSB-CoA, ATP is hydrolyzed to AMP and PPi. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C11H10O6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 238.196 Monoisotopic: 238.048835202 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | QJYRAJSESKVEAE-PSASIEDQSA-L | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C11H12O6/c12-7(4-5-9(14)15)6-2-1-3-8(13)10(6)11(16)17/h1-3,8,10,13H,4-5H2,(H,14,15)(H,16,17)/p-2/t8-,10-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (1R,6R)-2-(3-carboxypropanoyl)-6-hydroxycyclohexa-2,4-diene-1-carboxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | shchc | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H][C@@]1(O)C=CC=C(C(=O)CCC([O-])=O)[C@@]1([H])C([O-])=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as gamma-keto acids and derivatives. These are organic compounds containing an aldehyde substituted with a keto group on the C4 carbon atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Gamma-keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Gamma-keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic homomonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | (1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate <> Water + 2-Succinylbenzoate 2-Succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate <> (1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate + Pyruvic acid 2-Succinylbenzoate + Water <> (1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate (1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate > 2-Succinylbenzoate + Water 2-Succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate > (1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate + Pyruvic acid 2-Succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate > (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate + Pyruvic acid + (1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate + (1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate > 2-succinylbenzoate + Water + 2-Succinylbenzoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Converts SHCHC to OSB
- Gene Name:
- menC
- Uniprot ID:
- P29208
- Molecular weight:
- 35476
Reactions
| (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate = 2-succinylbenzoate + H(2)O. |
- General function:
- Involved in 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase activity
- Specific function:
- Catalyzes a proton abstraction reaction that results in 2,5-elimination of pyruvate from 2-succinyl-5-enolpyruvyl-6- hydroxy-3-cyclohexene-1-carboxylate (SEPHCHC) and the formation of 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate (SHCHC). Is also able to catalyze the hydrolysis of the thioester bond in palmitoyl-CoA in vitro
- Gene Name:
- menH
- Uniprot ID:
- P37355
- Molecular weight:
- 27682
Reactions
| 5-enolpyruvoyl-6-hydroxy-2-succinylcyclohex-3-ene-1-carboxylate = (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate + pyruvate. |