| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:21:21 -0600 |
|---|
| Update Date | 2015-06-03 17:19:04 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | L-Aspartyl-4-phosphate |
|---|
| Description | L-Aspartyl-4-phosphate is involved in both the lysine biosynthesis and homoserine biosynthesis pathways. |
|---|
| Structure | |
|---|
| Synonyms: | - 4-Phospho-L-aspartate
- 4-Phospho-L-aspartic acid
- Asp-P
- L-β-aspartyl-P
- L-β-aspartyl-phosphate
- L-β-aspartyl-phosphoric acid
- L-4-Aspartyl phosphate
- L-4-Aspartyl phosphoric acid
- L-Aspartate b-semialdehyde
- L-Aspartate beta-semialdehyde
- L-Aspartate β-semialdehyde
- L-Aspartic acid b-semialdehyde
- L-Aspartic acid beta-semialdehyde
- L-Aspartic acid β-semialdehyde
- L-aspartyl-β-phosphate
- L-Aspartyl-β-phosphoric acid
- L-aspartyl-4-P
- L-Aspartyl-4-phosphoric acid
- L-aspartyl-b-phosphate
- L-Aspartyl-b-phosphoric acid
- L-Aspartyl-beta-phosphate
- L-Aspartyl-beta-phosphoric acid
- L-Aspartyl-β-phosphate
- L-Aspartyl-β-phosphoric acid
- L-b-aspartyl-P
- L-b-aspartyl-phosphate
- L-b-Aspartyl-phosphoric acid
- L-beta-Aspartyl-P
- L-beta-Aspartyl-phosphate
- L-beta-Aspartyl-phosphoric acid
- L-β-Aspartyl-P
- L-β-Aspartyl-phosphate
- L-β-Aspartyl-phosphoric acid
|
|---|
| Chemical Formula: | C4H8NO7P |
|---|
| Weight: | Average: 213.0826
Monoisotopic: 213.003838127 |
|---|
| InChI Key: | IXZNKTPIYKDIGG-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H8NO7P/c5-2(4(7)8)1-3(6)12-13(9,10)11/h2H,1,5H2,(H,7,8)(H2,9,10,11) |
|---|
| CAS number: | 22138-53-0 |
|---|
| IUPAC Name: | 2-amino-4-oxo-4-(phosphonooxy)butanoic acid |
|---|
| Traditional IUPAC Name: | aspartyl phosphate |
|---|
| SMILES: | NC(CC(=O)OP(O)(O)=O)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aspartic acid and derivatives. Aspartic acid and derivatives are compounds containing an aspartic acid or a derivative thereof resulting from reaction of aspartic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Aspartic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aspartic acid or derivatives
- Alpha-amino acid
- Acyl monophosphate
- Acyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Fatty acid
- Carboxylic acid salt
- Amino acid
- Carboxylic acid
- Organic salt
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Primary aliphatic amine
- Organopnictogen compound
- Organic nitrogen compound
- Carbonyl group
- Amine
- Organic oxygen compound
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Lysine biosynthesis | PW000771 | 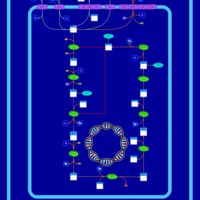   | | Secondary Metabolites: threonine biosynthesis from aspartate | PW000976 | 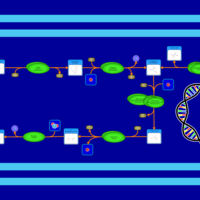  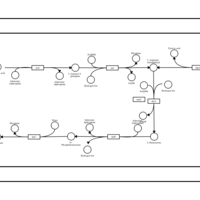 | | threonine biosynthesis | PW000817 | 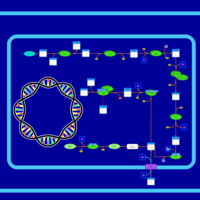 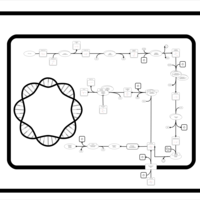 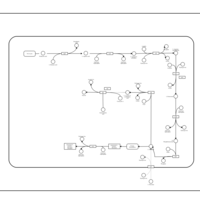 |
|
|---|
| KEGG Pathways: | - Cysteine and methionine metabolism ec00270
- Glycine, serine and threonine metabolism ec00260
- Lysine biosynthesis ec00300
- Metabolic pathways eco01100
- Microbial metabolism in diverse environments ec01120
- Monobactam biosynthesis eco00261
|
|---|
| EcoCyc Pathways: | |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|
