Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
L-Fuculose (M2MDB000595)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 14:04:10 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-10-15 16:14:30 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | L-Fuculose | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | L-fuculose is a member of the chemical class known as Hexoses. These are monosaccharides in which the sugar unit is a hexose. Fuculose or 6-deoxy-tagatose is a ketohexose deoxy sugar. (WikiPedia) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
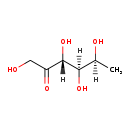
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H12O5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 164.157 Monoisotopic: 164.068473486 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | QZNPNKJXABGCRC-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H12O5/c1-3(8)5(10)6(11)4(9)2-7/h3,5-8,10-11H,2H2,1H3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 2438-80-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (3R,4R,5S)-1,3,4,5-tetrahydroxyhexan-2-one | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | L-fuculose | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(O)C(O)C(O)C(=O)CO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as fatty alcohols. These are aliphatic alcohols consisting of a chain of a least six carbon atoms. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Fatty Acyls | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Fatty alcohols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Fatty alcohols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 150 - 153 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | L-Fucose <> L-Fuculose Adenosine triphosphate + L-Fuculose <> ADP + L-Fuculose 1-phosphate + Hydrogen ion Adenosine triphosphate + L-Fuculose <> ADP + L-Fuculose 1-phosphate L-fucose <> L-Fuculose L-Fuculose + Adenosine triphosphate > Hydrogen ion + L-Fuculose 1-phosphate + ADP L-Fucose > L-Fuculose Adenosine triphosphate + L-Fuculose > ADP + L-Fuculose 1-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in phosphotransferase activity, alcohol group as acceptor
- Specific function:
- ATP + L-fuculose = ADP + L-fuculose 1- phosphate
- Gene Name:
- fucK
- Uniprot ID:
- P11553
- Molecular weight:
- 53235
Reactions
| ATP + L-fuculose = ADP + L-fuculose 1-phosphate. |
- General function:
- Involved in L-fucose isomerase activity
- Specific function:
- Converts the aldose L-fucose into the corresponding ketose L-fuculose. Is also able to convert D-arabinose into D- ribulose, but this isomerase has a higher affinity for fucose and fuculose than for arabinose and ribulose, respectively
- Gene Name:
- fucI
- Uniprot ID:
- P69922
- Molecular weight:
- 64976
Reactions
| L-fucose = L-fuculose. |
Transporters
- General function:
- Involved in transmembrane transport
- Specific function:
- Involved in the efflux of sugars. The physiological role may be the detoxification of non-metabolizable sugar analogs
- Gene Name:
- setC
- Uniprot ID:
- P31436
- Molecular weight:
- 43493