| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 13:55:15 -0600 |
|---|
| Update Date | 2015-06-03 15:17:57 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Tiglyl-CoA |
|---|
| Description | Tiglyl-CoA is a metabolite in the degradation of isoleucine to propionic acid pathway. It is a substrate of enoyl-CoA hydratase-isomerase [EC:4.2.1.17] and converted to (2S,3S)-3-Hydroxy-2-methylbutanoyl-CoA. |
|---|
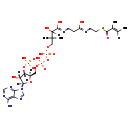
| Structure | |
|---|
| Synonyms: | - (E)-2-Methylcrotonoyl-CoA
- (E)-2-Methylcrotonoyl-Coenzyme A
- 2-Methylbut-2-enoyl-CoA
- 2-Methylbut-2-enoyl-Coenzyme A
- 2-Methylcrotanoyl-CoA
- 2-Methylcrotanoyl-Coenzyme A
- 2-Methylcrotonoyl-CoA
- 2-Methylcrotonoyl-Coenzyme A
- 2-Methylcrotonyl-CoA
- 2-Methylcrotonyl-Coenzyme A
- Methylcrotonoyl-CoA
- Methylcrotonoyl-Coenzyme A
- Methylcrotonyl-CoA
- Methylcrotonyl-Coenzyme A
- Tigloyl-CoA
- Tigloyl-Coenzyme A
- Tiglyl-CoA
- Tiglyl-coenzyme A
- Trans-2-Methylbut-2-enoyl-CoA
- Trans-2-Methylbut-2-enoyl-Coenzyme A
|
|---|
| Chemical Formula: | C26H42N7O17P3S |
|---|
| Weight: | Average: 849.635
Monoisotopic: 849.157073179 |
|---|
| InChI Key: | PMWATMXOQQZNBX-APMDNKNFSA-N |
|---|
| InChI: | InChI=1S/C26H42N7O17P3S/c1-5-14(2)25(38)54-9-8-28-16(34)6-7-29-23(37)20(36)26(3,4)11-47-53(44,45)50-52(42,43)46-10-15-19(49-51(39,40)41)18(35)24(48-15)33-13-32-17-21(27)30-12-31-22(17)33/h5,12-13,15,18-20,24,35-36H,6-11H2,1-4H3,(H,28,34)(H,29,37)(H,42,43)(H,44,45)(H2,27,30,31)(H2,39,40,41)/b14-5+/t15-,18-,19-,20?,24-/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-2,2-dimethyl-3-({2-[(2-{[(2E)-2-methylbut-2-enoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)propoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional IUPAC Name: | [(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-2-({[hydroxy([hydroxy(3-hydroxy-2,2-dimethyl-3-({2-[(2-{[(2E)-2-methylbut-2-enoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)propoxy)phosphoryl]oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES: | C\C=C(/C)C(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C(N)N=CN=C12 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ortho cresols. These are organic compounds containing an ortho-cresol moiety, which consists of a benzene bearing one hydroxyl group at ring positions 1 and 2, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Cresols |
|---|
| Direct Parent | Ortho cresols |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-cresol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Toluene
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | -4 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | - Valine, leucine and isoleucine degradation ec00280
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Duran M, Bruinvis L, Ketting D, Kamerling JP, Wadman SK, Schutgens RB: The identification of (E)-2-methylglutaconic acid, a new isoleucine metabolite, in the urine of patients with beta-ketothiolase deficiency, propionic acidaemia and methylmalonic acidaemia. Biomed Mass Spectrom. 1982 Jan;9(1):1-5. Pubmed: 7059658
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|