| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 13:50:14 -0600 |
|---|
| Update Date | 2015-06-03 15:53:56 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Glutaryl-CoA |
|---|
| Description | Glutaryl-coenzyme A (or Glutaryl-CoA) is an intermediate in the metabolism of lysine and tryptophan. |
|---|
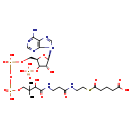
| Structure | |
|---|
| Synonyms: | - Glutaryl-CoA
- Glutaryl-coenzyme A
|
|---|
| Chemical Formula: | C26H42N7O19P3S |
|---|
| Weight: | Average: 881.633
Monoisotopic: 881.146902423 |
|---|
| InChI Key: | SYKWLIJQEHRDNH-KRPIADGTSA-N |
|---|
| InChI: | InChI=1S/C26H42N7O19P3S/c1-26(2,21(39)24(40)29-7-6-15(34)28-8-9-56-17(37)5-3-4-16(35)36)11-49-55(46,47)52-54(44,45)48-10-14-20(51-53(41,42)43)19(38)25(50-14)33-13-32-18-22(27)30-12-31-23(18)33/h12-14,19-21,25,38-39H,3-11H2,1-2H3,(H,28,34)(H,29,40)(H,35,36)(H,44,45)(H,46,47)(H2,27,30,31)(H2,41,42,43)/t14-,19-,20-,21?,25-/m1/s1 |
|---|
| CAS number: | 103192-48-9 |
|---|
| IUPAC Name: | 5-{[2-(3-{3-[({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-2-hydroxy-3-methylbutanamido}propanamido)ethyl]sulfanyl}-5-oxopentanoic acid |
|---|
| Traditional IUPAC Name: | 5-({2-[3-(3-{[({[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-2-hydroxy-3-methylbutanamido)propanamido]ethyl}sulfanyl)-5-oxopentanoic acid |
|---|
| SMILES: | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N)C(O)C(=O)NCCC(=O)NCCSC(=O)CCCC(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as long-chain fatty acyl coas. These are acyl CoAs where the group acylated to the coenzyme A moiety is a long aliphatic chain of 13 to 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | Long-chain fatty acyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Monosaccharide
- Pyrimidine
- Alkyl phosphate
- Fatty amide
- Phosphoric acid ester
- Tetrahydrofuran
- Imidazole
- Azole
- Heteroaromatic compound
- Carbothioic s-ester
- Secondary alcohol
- Thiocarboxylic acid ester
- Carboxamide group
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Organosulfur compound
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Primary amine
- Organopnictogen compound
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | -5 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | - Benzoate degradation via hydroxylation ec00362
- Fatty acid metabolism ec00071
- Lysine degradation ec00310
- Microbial metabolism in diverse environments ec01120
- Tryptophan metabolism ec00380
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-00kr-0005009000-eb3a971b38526182c8ac | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-0a4i-0269300000-b433e45be2e4ddb9b3dd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-05n1-0009700000-d04f275432ce90be4c03 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-0a4l-0691000000-1c9c9dcd3392e14e12d9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-0f80-0009841000-c64bfcd1084f04509fc5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-000i-0009000000-8ef4f57ae35ca24154ac | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-014r-0001920000-f105e4c7bcf62b49d748 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 61V, negative | splash10-001i-0000100090-7e0c0d1ae140f9f6c57c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 77V, negative | splash10-053r-0000920060-2e8ff86ba386000c087e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 100V, negative | splash10-0a6r-1211920000-b87d792a259c80a7b01d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 123V, negative | splash10-056r-9835710000-f9df91f65d08757ed109 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 146V, negative | splash10-004i-9512100000-6725b9c211e210ffa4f9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 169V, negative | splash10-004i-9400000000-3b7b4c8aa8db05827423 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 200V, negative | splash10-004i-9300000000-a481ad1113e5856425ad | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 232V, negative | splash10-004i-9200000000-484261819d8fe28a06bc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 278V, negative | splash10-004i-9100000000-1ced5b391be9b0f5176c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-001i-0001960370-e5f5990aef17b3100ae7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-00di-0190000000-0bdc30775338012cc42f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 61V, negative | splash10-0a4i-0900000000-816bc86479bd970ceed6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1912000130-9b9c96863df04861fbee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0913000000-43f2e61c32509ed14b97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1901000000-4ff7fbc1bd490ad4c020 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01q9-3911030350-425e74ceb7a0a4256402 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01si-2901010010-f93605fcb2512efdd305 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-6900100000-74a35eb8b1b0810315e4 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Corral I, Martinez Castrillo JC, Martinez-Pardo M, Gimeno A: [Glutaric aciduria type I: diagnosis in adulthood and phenotypic variability] Neurologia. 2001 Oct;16(8):377-80. Pubmed: 11738016
- Hyman DB, Tanaka K: Specific glutaryl-CoA dehydrogenating activity is deficient in cultured fibroblasts from glutaric aciduria patients. J Clin Invest. 1984 Mar;73(3):778-84. Pubmed: 6423663
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Mahfoud A, Dominguez CL, Rizzo C, Ribes A: [In utero macrocephaly as clinical manifestation of glutaric aciduria type I. Report of a novel mutation] Rev Neurol. 2004 Nov 16-30;39(10):939-42. Pubmed: 15573311
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|