Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
Pyridoxine 5'-phosphate (M2MDB000338)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 13:49:55 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-06-03 15:53:56 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Pyridoxine 5'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Pyridoxine 5'-phosphate is a substrate for Pyridoxine-5'-phosphate oxidase and Pyridoxal kinase. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
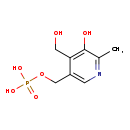
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C8H12NO6P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 249.1577 Monoisotopic: 249.040223633 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | WHOMFKWHIQZTHY-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C8H12NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2,10-11H,3-4H2,1H3,(H2,12,13,14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 447-05-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[5-hydroxy-4-(hydroxymethyl)-6-methylpyridin-3-yl]methoxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | pyridoxine-5'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=NC=C(COP(O)(O)=O)C(CO)=C1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as pyridoxine-5'-phosphates. These are pyridoxines that carry a phosphate group at the 5-position. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyridines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyridoxines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyridoxine-5'-phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 1-Deoxy-D-xylulose 5-phosphate + NAD + O-Phospho-4-hydroxy-L-threonine > Carbon dioxide + Hydrogen ion +2 Water + NADH + Pyridoxine 5'-phosphate + Phosphate Water + Pyridoxine 5'-phosphate > Phosphate + Pyridoxine Oxygen + Pyridoxine 5'-phosphate > Hydrogen peroxide + Pyridoxal 5'-phosphate Adenosine triphosphate + Pyridoxine > ADP + Hydrogen ion + Pyridoxine 5'-phosphate Pyridoxine 5'-phosphate + Oxygen <> Hydrogen peroxide + Pyridoxal 5'-phosphate Adenosine triphosphate + Pyridoxine <> ADP + Pyridoxine 5'-phosphate 3-Amino-2-oxopropyl phosphate + 1-Deoxy-D-xylulose 5-phosphate + 3-Amino-2-oxopropyl phosphate <> Pyridoxine 5'-phosphate + Phosphate +2 Water 1-Amino-propan-2-one-3-phosphate + 1-Deoxy-D-xylulose 5-phosphate > Hydrogen ion + Pyridoxine 5'-phosphate + Phosphate + Water 1-Deoxy-D-xylulose 5-phosphate + 2-Amino-3-phosphonopropionic acid > Pyridoxine 5'-phosphate + Inorganic phosphate +2 Water Pyridoxamine 5'-phosphate + Water + Oxygen + Pyridoxine 5'-phosphate <> Pyridoxal 5'-phosphate + Ammonia + Hydrogen peroxide 1-Deoxy-D-xylulose 5-phosphate + 2-Amino-3-phosphonopropionic acid + 1-Deoxy-D-xylulose 5-phosphate + 2-Amino-3-phosphonopropionic acid > Pyridoxine 5'-phosphate + Phosphate + Hydrogen ion +2 Water Pyridoxine + Adenosine triphosphate > Pyridoxine 5'-phosphate + Adenosine diphosphate + Hydrogen ion + ADP 3 3-Amino-2-oxopropyl phosphate + 1-Deoxy-D-xylulose 5-phosphate <> Pyridoxine 5'-phosphate + Phosphate +2 Water Oxygen + Pyridoxine 5'-phosphate > Hydrogen peroxide + Pyridoxal 5'-phosphate Oxygen + Pyridoxine 5'-phosphate > Hydrogen peroxide + Pyridoxal 5'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Argoudelis, Chris J. Preparation of crystalline pyridoxine 5'-phosphate and some of its properties. Journal of Agricultural and Food Chemistry (1986), 34(6), 995-8. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the complicated ring closure reaction between the two acyclic compounds 1-deoxy-D-xylulose-5-phosphate (DXP) and 3-amino-2-oxopropyl phosphate (1-amino-acetone-3-phosphate or AAP) to form pyridoxine 5'-phosphate (PNP) and inorganic phosphate
- Gene Name:
- pdxJ
- Uniprot ID:
- P0A794
- Molecular weight:
- 26384
Reactions
| 1-deoxy-D-xylulose 5-phosphate + 3-amino-2-oxopropyl phosphate = pyridoxine 5'-phosphate + phosphate + 2 H(2)O. |
- General function:
- Involved in 4-hydroxythreonine-4-phosphate dehydrogenase activity
- Specific function:
- Catalyzes the NAD(P)-dependent oxidation of 4- (phosphohydroxy)-L-threonine (HTP) into 2-amino-3-oxo-4- (phosphohydroxy)butyric acid which spontaneously decarboxylates to form 3-amino-2-oxopropyl phosphate (AHAP)
- Gene Name:
- pdxA
- Uniprot ID:
- P19624
- Molecular weight:
- 35114
Reactions
| 4-(phosphonooxy)-L-threonine + NAD(+) = (2S)-2-amino-3-oxo-4-phosphonooxybutanoate + NADH. |
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the dephosphorylation of the artificial chromogenic substrate p-nitrophenyl phosphate (pNPP) and of the natural substrates pyridoxalphosphate and erythrose 4-phosphate
- Gene Name:
- ybhA
- Uniprot ID:
- P21829
- Molecular weight:
- 30201
- General function:
- Involved in pyridoxal kinase activity
- Specific function:
- Phosphorylates B6 vitamers; functions in a salvage pathway. Uses pyridoxal, pyridoxine, and pyridoxamine as substrates
- Gene Name:
- pdxK
- Uniprot ID:
- P40191
- Molecular weight:
- 30847
Reactions
| ATP + pyridoxal = ADP + pyridoxal 5'-phosphate. |
- General function:
- Involved in pyridoxal kinase activity
- Specific function:
- Phosphorylates B6 vitamers; functions in a salvage pathway. Uses pyridoxamine, but has negligible activity toward pyridoxal and pyridoxine as substrates
- Gene Name:
- pdxY
- Uniprot ID:
- P77150
- Molecular weight:
- 31322
Reactions
| ATP + pyridoxal = ADP + pyridoxal 5'-phosphate. |
- General function:
- Coenzyme transport and metabolism
- Specific function:
- Catalyzes the oxidation of either pyridoxine 5'- phosphate (PNP) or pyridoxamine 5'-phosphate (PMP) into pyridoxal 5'-phosphate (PLP)
- Gene Name:
- pdxH
- Uniprot ID:
- P0AFI7
- Molecular weight:
- 25545
Reactions
| Pyridoxamine 5'-phosphate + H(2)O + O(2) = pyridoxal 5'-phosphate + NH(3) + H(2)O(2). |
| Pyridoxine 5'-phosphate + O(2) = pyridoxal 5'-phosphate + H(2)O(2). |