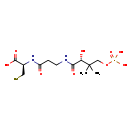Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
4-Phosphopantothenoylcysteine (M2MDB000259)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 13:45:29 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-06-03 15:53:45 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 4-Phosphopantothenoylcysteine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 4-Phosphopantothenoylcysteine (PPC) is an intermediate in the biosynthetic machinery (pathway) that converts pantothenate (vitamin B5) into coenzyme A (CoA). The enzyme Phosphopantothenoylcysteine decarboxylase catalyzes the decarboxylation of PPC to 4'-phosphopantetheine. Coenzyme A is the principal acyl carrier and is required for many synthetic and degradative reactions in intermediary metabolism, and is an essential cofactor in all living systems. (PMID: 15450493, 16371361, 14501115) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C12H23N2O9PS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 402.358 Monoisotopic: 402.086187546 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | XQYALQVLCNHCFT-CBAPKCEASA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C12H23N2O9PS/c1-12(2,6-23-24(20,21)22)9(16)10(17)13-4-3-8(15)14-7(5-25)11(18)19/h7,9,16,25H,3-6H2,1-2H3,(H,13,17)(H,14,15)(H,18,19)(H2,20,21,22)/t7-,9-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 7196-09-0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2R)-2-{3-[(2R)-2-hydroxy-3-methyl-3-[(phosphonooxy)methyl]butanamido]propanamido}-3-sulfanylpropanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (2R)-2-{3-[(2R)-2-hydroxy-3-methyl-3-[(phosphonooxy)methyl]butanamido]propanamido}-3-sulfanylpropanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(C)(COP(O)(O)=O)[C@@H](O)C(=O)NCCC(=O)N[C@@H](CS)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as hybrid peptides. Hybrid peptides are compounds containing at least two different types of amino acids (alpha, beta, gamma, delta) linked to each other through a peptide bond. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Peptidomimetics | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Hybrid peptides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Hybrid peptides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | D-4'-Phosphopantothenate + Cytidine triphosphate + L-Cysteine > 4-Phosphopantothenoylcysteine + Cytidine monophosphate + Hydrogen ion + Pyrophosphate 4-Phosphopantothenoylcysteine + Hydrogen ion <> Carbon dioxide + Pantetheine 4'-phosphate + pantotheine 4'-phosphate 4-Phosphopantothenoylcysteine <> Pantetheine 4'-phosphate + Carbon dioxide Adenosine triphosphate + D-4'-Phosphopantothenate + L-Cysteine <> Adenosine monophosphate + Pyrophosphate + 4-Phosphopantothenoylcysteine Cytidine triphosphate + D-4'-Phosphopantothenate + L-Cysteine <> Cytidine monophosphate + Pyrophosphate + 4-Phosphopantothenoylcysteine Adenosine triphosphate + D-Pantothenoyl-L-cysteine <> ADP + 4-Phosphopantothenoylcysteine Hydrogen ion + 4-Phosphopantothenoylcysteine > Pantetheine 4'-phosphate + Carbon dioxide 4-Phosphopantothenoylcysteine > pantotheine 4'-phosphate + Carbon dioxide Cytidine triphosphate + (R)-4'-phosphopantothenate + L-Cysteine > Cytidine monophosphate + Pyrophosphate + 4-Phosphopantothenoylcysteine Adenosine triphosphate + D-Pantothenoyl-L-cysteine > Adenosine diphosphate + 4-Phosphopantothenoylcysteine + ADP Cytidine triphosphate + D-4'-Phosphopantothenate + L-Cysteine + D-4'-Phosphopantothenate > Cytidine monophosphate + Pyrophosphate + 4-Phosphopantothenoylcysteine + Cytidine monophosphate 4-Phosphopantothenoylcysteine > Pantetheine 4'-phosphate + Carbon dioxide + pantotheine 4'-phosphate D-4'-Phosphopantothenate + Cytidine triphosphate + L-Cysteine + D-4'-Phosphopantothenate > Cytidine monophosphate + Pyrophosphate + Hydrogen ion + 4-Phosphopantothenoylcysteine + Cytidine monophosphate 4-Phosphopantothenoylcysteine + Hydrogen ion > Carbon dioxide + 4'-phosphopantetheine + 4'-phosphopantetheine D-4'-Phosphopantothenate + Cytidine triphosphate + L-Cysteine >4 4-Phosphopantothenoylcysteine + Cytidine monophosphate + Hydrogen ion + Pyrophosphate D-4'-Phosphopantothenate + Cytidine triphosphate + L-Cysteine >4 4-Phosphopantothenoylcysteine + Cytidine monophosphate + Hydrogen ion + Pyrophosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in pantothenate kinase activity
- Specific function:
- ATP + (R)-pantothenate = ADP + (R)-4'- phosphopantothenate
- Gene Name:
- coaA
- Uniprot ID:
- P0A6I3
- Molecular weight:
- 36359
Reactions
| ATP + (R)-pantothenate = ADP + (R)-4'-phosphopantothenate. |
- General function:
- Involved in phosphopantothenate--cysteine ligase activity
- Specific function:
- Catalyzes two steps in the biosynthesis of coenzyme A. In the first step cysteine is conjugated to 4'-phosphopantothenate to form 4-phosphopantothenoylcysteine, in the latter compound is decarboxylated to form 4'-phosphopantotheine
- Gene Name:
- coaBC
- Uniprot ID:
- P0ABQ0
- Molecular weight:
- 43438
Reactions
| N-((R)-4'-phosphopantothenoyl)-L-cysteine = pantotheine 4'-phosphate + CO(2). |
| CTP + (R)-4'-phosphopantothenate + L-cysteine = CMP + diphosphate + N-((R)-4'-phosphopantothenoyl)-L-cysteine. |