| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 13:45:23 -0600 |
|---|
| Update Date | 2015-09-17 15:41:09 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
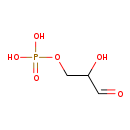
| Name: | D-Glyceraldehyde 3-phosphate |
|---|
| Description | Glyceraldehyde 3-phosphate (G3P) or triose phosphate is an aldotriose, an important metabolic intermediate in both glycolysis and gluconeogenesis, and in tryptophan biosynthesis. G3P is formed from Fructose-1,6-bisphosphate, Dihydroxyacetone phosphate (DHAP),and 1,3-bisphosphoglycerate, (1,3BPG), and this is how glycerol (as DHAP) enters the glycolytic and gluconeogenesis pathways. |
|---|
| Structure | |
|---|
| Synonyms: | - (2R)-2-hydroxy-3-(phosphonooxy)-propanal
- (2R)-2-hydroxy-3-(phosphonooxy)-propanal
- 2-Hydroxy-3-(phosphonooxy)-Propanal
- 3-Phosphoglyceraldehyde
- D-Glyceraldehyde 3-phosphate
- D-Glyceraldehyde 3-phosphoric acid
- D-Glyceraldehyde-3-P
- DL-Glyceraldehyde 3-phosphate
- DL-Glyceraldehyde 3-phosphoric acid
- GAP
- Glyceraldehyde 3-phosphate
- Glyceraldehyde 3-phosphoric acid
- Glyceraldehyde-3-P
- Glyceraldehyde-3-phosphate
- Glyceraldehyde-3-phosphoric acid
- Glyceraldehyde-P
- Glyceraldehyde-phosphate
- Glyceraldehyde-phosphoric acid
- Triose phosphate
- Triose phosphoric acid
|
|---|
| Chemical Formula: | C3H7O6P |
|---|
| Weight: | Average: 170.0578
Monoisotopic: 169.998024468 |
|---|
| InChI Key: | LXJXRIRHZLFYRP-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C3H7O6P/c4-1-3(5)2-9-10(6,7)8/h1,3,5H,2H2,(H2,6,7,8) |
|---|
| CAS number: | 142-10-9 |
|---|
| IUPAC Name: | (2-hydroxy-3-oxopropoxy)phosphonic acid |
|---|
| Traditional IUPAC Name: | glyceraldehyde 3 phosphate |
|---|
| SMILES: | OC(COP(O)(O)=O)C=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glyceraldehyde-3-phosphates. Glyceraldehyde-3-phosphates are compounds containing a glyceraldehyde substituted at position O3 by a phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Glyceraldehyde-3-phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glyceraldehyde-3-phosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Alpha-hydroxyaldehyde
- Secondary alcohol
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Aldehyde
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | 2-Keto-3-deoxy-6-phosphogluconic acid > D-Glyceraldehyde 3-phosphate + Pyruvic acidD-Tagatose 1,6-bisphosphate <> Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphateFructose 1,6-bisphosphate > Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphateDeoxyribose 5-phosphate > D-Glyceraldehyde 3-phosphate + Acetaldehyde2-Dehydro-3-deoxy-D-galactonate 6-phosphate > Pyruvic acid + D-Glyceraldehyde 3-phosphatePyruvic acid + D-Glyceraldehyde 3-phosphate > 1-Deoxy-D-xylulose 5-phosphate + Carbon dioxideFructose 6-phosphate > Dihydroxyacetone + D-Glyceraldehyde 3-phosphateD-Glyceraldehyde 3-phosphate + Inorganic phosphate + NAD > 3-phospho-D-glyceroyl phosphate + NADHD-Tagatose 1,6-bisphosphate > Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphateSedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate > D-Erythrose 4-phosphate + Fructose 6-phosphateSedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate > D-Ribose-5-phosphate + Xylulose 5-phosphateD-Glyceraldehyde 3-phosphate > Dihydroxyacetone phosphateL-Serine + Indoleglycerol phosphate + Indole <> L-Tryptophan + D-Glyceraldehyde 3-phosphate + WaterD-Glyceraldehyde 3-phosphate + Pyruvic acid + Hydrogen ion + D-Glyceraldehyde 3-phosphate > 1-Deoxy-D-xylulose 5-phosphate + Carbon dioxide + 1-Deoxy-D-xylulose 5-phosphateD-Glyceraldehyde 3-phosphate + Hydrogen ion + D-Glyceraldehyde 3-phosphate > Carbon dioxide + 1-Deoxy-D-xylulose 5-phosphate + 1-Deoxy-D-xylulose 5-phosphateXylulose 5-phosphate + D-Ribose-5-phosphate + Xylulose 5-phosphate <> D-Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate + D-Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphateD-Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate + D-Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate <> beta-D-Fructose 6-phosphate + D-Erythrose 4-phosphateFructose 1,6-bisphosphate + Fructose 1,6-bisphosphate <> D-Glyceraldehyde 3-phosphate + Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphateD-Glyceraldehyde 3-phosphate + NAD + Phosphate + D-Glyceraldehyde 3-phosphate > Glyceric acid 1,3-biphosphate + NADH + Hydrogen ion + Glyceric acid 1,3-biphosphateGlyceric acid 1,3-biphosphate + NADH + Hydrogen ion + Glyceric acid 1,3-biphosphate > NAD + Phosphate + D-Glyceraldehyde 3-phosphate + D-Glyceraldehyde 3-phosphate(1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate + (1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate > D-Glyceraldehyde 3-phosphate + Indole + D-Glyceraldehyde 3-phosphateD-Glyceraldehyde 3-phosphate + D-Glyceraldehyde 3-phosphate <> Dihydroxyacetone phosphateD-Glyceraldehyde 3-phosphate + Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate > Fructose 1,6-bisphosphate + Fructose 1,6-bisphosphateFructose 1,6-bisphosphate + Fructose 1,6-bisphosphate > Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate + D-Glyceraldehyde 3-phosphate2-dehydro-3-deoxy-D-galactonate 6-phosphate + 2-Dehydro-3-deoxy-D-galactonate 6-phosphate > Pyruvic acid + D-Glyceraldehyde 3-phosphate + D-Glyceraldehyde 3-phosphateD-tagatofuranose 1,6-bisphosphate > Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate + D-Glyceraldehyde 3-phosphate2-Keto-3-deoxy-6-phosphogluconic acid > D-Glyceraldehyde 3-phosphate + Pyruvic acid + D-Glyceraldehyde 3-phosphateDeoxyribose 5-phosphate > Acetaldehyde + D-Glyceraldehyde 3-phosphateD-Erythrose 4-phosphate + Xylulose 5-phosphate <> Fructose 6-phosphate + D-Glyceraldehyde 3-phosphateFructose 6-phosphate + D-Glyceraldehyde 3-phosphate <> D-Erythrose 4-phosphate + Xylulose 5-phosphateSedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate <> D-Erythrose 4-phosphate + beta-D-Fructose 6-phosphateD-Glyceraldehyde 3-phosphate + Hydrogen ion + Pyruvic acid <> Carbon dioxide + 1-Deoxy-D-xylulose 5-phosphatePyruvic acid + D-Glyceraldehyde 3-phosphate <> 1-Deoxy-D-xylulose 5-phosphate + Carbon dioxideDihydroxyacetone phosphate <> D-Glyceraldehyde 3-phosphateD-Glyceraldehyde 3-phosphate <> Dihydroxyacetone phosphateD-Erythrose 4-phosphate + Xylulose 5-phosphate <> Fructose 6-phosphate + D-Glyceraldehyde 3-phosphateSedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate <> D-Erythrose 4-phosphate + beta-D-Fructose 6-phosphateD-Glyceraldehyde 3-phosphate + Hydrogen ion + Pyruvic acid <> Carbon dioxide + 1-Deoxy-D-xylulose 5-phosphateDihydroxyacetone phosphate <> D-Glyceraldehyde 3-phosphate More...
|
|---|
| SMPDB Pathways: | | Galactitol and galactonate degradation | PW000820 |    | | Galactose metabolism | PW000821 |    | | Gluconeogenesis from L-malic acid | PW000819 |    | | Pentose Phosphate | PW000893 |    | | Secondary metabolites: isoprenoid biosynthesis (nonmevalonate pathway) | PW000975 |    | | Secondary metabolites: methylerythritol phosphate and polyisoprenoid biosynthesis | PW000958 |    | | Thiazole Biosynthesis I | PW002041 |    | | Tryptophan metabolism | PW000815 |    | | Vitamin B6 1430936196 | PW000891 |    | | fructose metabolism | PW000913 |    | | glycerol metabolism | PW000914 |    | | glycerol metabolism II | PW000915 |    | | glycerol metabolism III (sn-glycero-3-phosphoethanolamine) | PW000916 |    | | glycerol metabolism IV (glycerophosphoglycerol) | PW000917 |    | | glycerol metabolism V (glycerophosphoserine) | PW000918 |    | | glycolysis and pyruvate dehydrogenase | PW000785 |    | | hexuronide and hexuronate degradation | PW000834 |    | | ketogluconate metabolism | PW002003 |    | | purine deoxyribonucleosides degradation | PW002077 |    | | tryptophan metabolism II | PW001916 |    |
|
|---|
| KEGG Pathways: | - Arginine and proline metabolism ec00330
- Biosynthesis of ansamycins ec01051
- Carbon fixation in photosynthetic organisms ec00710
- Fructose and mannose metabolism ec00051
- Galactose metabolism ec00052
- Glycine, serine and threonine metabolism ec00260
- Glycolysis / Gluconeogenesis ec00010
- Glyoxylate and dicarboxylate metabolism ec00630
- Inositol phosphate metabolism ec00562
- Metabolic pathways eco01100
- Methane metabolism ec00680
- Microbial metabolism in diverse environments ec01120
- Pentose and glucuronate interconversions ec00040
- Pentose phosphate pathway ec00030
- Phenylalanine, tyrosine and tryptophan biosynthesis ec00400
- Terpenoid backbone biosynthesis ec00900
- Thiamine metabolism ec00730
- Tryptophan metabolism ec00380
- Vitamin B6 metabolism ec00750
|
|---|
| EcoCyc Pathways: | |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0m5s-3952000000-862ad552658a2dad7631 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-03dj-2943000000-879f36e9ffb61cdb7e7e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0m5s-3952000000-862ad552658a2dad7631 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-03dj-2943000000-879f36e9ffb61cdb7e7e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9300000000-7c325d284fb0e3770da5 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00xs-9720000000-2a59591392eaadc8cb67 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-002b-9000000000-6e7cef3b048204ad747c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 0V, positive | splash10-00di-1900000000-f7a7b256e1a0b030f168 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 0V, positive | splash10-00di-2900000000-eacc8d6b246e46b2340f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 1V, positive | splash10-00dj-4900000000-a66af0aa75aba70cb82a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 1V, positive | splash10-006t-7900000000-8b419c2e8472f9f74a49 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, positive | splash10-006t-9600000000-6504648a31d96ef44d49 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, positive | splash10-0002-9400000000-5a167503e18109d4af5b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, positive | splash10-0002-9200000000-76cd396626689c9235be | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, positive | splash10-0002-9100000000-9b592d8b0cba5a7af4c8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 4V, positive | splash10-0002-9100000000-7a7901afb70f6d8627c9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 4V, positive | splash10-0002-9000000000-bcb4ecbf7bc0e3ce3164 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 5V, positive | splash10-0002-9000000000-13cecd1b3662f69b69d2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 6V, positive | splash10-0002-9000000000-ad2b828477c8005d6ec0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 7V, positive | splash10-0002-9000000000-c4278124075952af95f1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 9V, positive | splash10-0002-9000000000-4387fd6f7164cef7bd72 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 11V, positive | splash10-0002-9000000000-4c200ed3c50ff96956b6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 13V, positive | splash10-0002-9000000000-c9b13016720f097cecbc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 16V, positive | splash10-0002-9000000000-f2389881e7483c78942f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 11V, positive | splash10-0005-9700000000-3e08f8453aeb088eee03 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-4900000000-416f405681faaf774486 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9300000000-e5728592219561b9203c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-eeb354dbde88d3794c6d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-016r-6900000000-50b19733dd05329d82c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-22bcce954e030ff911ed | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-5dbce0b156c269157c00 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: | Ballou, Clinton E.; Fischer, Hermann O. L. The synthesis of D-glyceraldehyde 3-phosphate. Journal of the American Chemical Society (1955), 77 3329-31. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|