| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 13:44:45 -0600 |
|---|
| Update Date | 2015-06-03 15:53:42 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | 5-Methyltetrahydropteroyltri-L-glutamic acid |
|---|
| Description | 5-methyltetrahydropteroyltri-L-glutamic acid is a member of the chemical class known as Biopterins and Derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative.5-Methyltetrahydropteroyltri-L-glutamate is formed under reaction between carbonyl group of 5-methyltetrahydropteroate and amine group on one end of three replicates of glutamate. It is involved in several pathways such as tetrahydrofolate biosynthesis and methionine biosynthesis. (YMDB00518) |
|---|
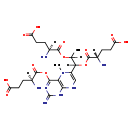
| Structure | |
|---|
| Synonyms: | - 5-Methyltetrahydropteroyltri-L-glutamate
- 5-Methyltetrahydropteroyltri-L-glutamic acid
- 5MTHTGlu
|
|---|
| Chemical Formula: | C25H36N8O12 |
|---|
| Weight: | Average: 640.5997
Monoisotopic: 640.245268656 |
|---|
| InChI Key: | SRBIKUAVFZRKSG-SUWBZHBXSA-N |
|---|
| InChI: | InChI=1S/C25H36N8O12/c1-10(43-22(40)11(26)3-6-15(34)35)19(44-23(41)12(27)4-7-16(36)37)14-9-30-20-18(33(14)2)21(32-25(29)31-20)45-24(42)13(28)5-8-17(38)39/h9-13,19H,3-8,26-28H2,1-2H3,(H,34,35)(H,36,37)(H,38,39)(H3,29,30,31,32)/t10?,11-,12-,13-,19?/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (4S)-4-amino-5-[(1-{[(2S)-2-amino-4-carboxybutanoyl]oxy}-1-(4-{[(2S)-2-amino-4-carboxybutanoyl]oxy}-2-imino-5-methyl-1,2,5,8-tetrahydropteridin-6-yl)propan-2-yl)oxy]-5-oxopentanoic acid |
|---|
| Traditional IUPAC Name: | (4S)-4-amino-5-[(1-{[(2S)-2-amino-4-carboxybutanoyl]oxy}-1-(4-{[(2S)-2-amino-4-carboxybutanoyl]oxy}-2-imino-5-methyl-1,8-dihydropteridin-6-yl)propan-2-yl)oxy]-5-oxopentanoic acid |
|---|
| SMILES: | [H][C@](N)(CCC(O)=O)C(=O)OC1=NC(=N)NC2=C1N(C)C(=CN2)C([H])(OC(=O)[C@@]([H])(N)CCC(O)=O)C([H])(C)OC(=O)[C@@]([H])(N)CCC(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hexacarboxylic acids and derivatives. These are carboxylic acids containing exactly six carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Hexacarboxylic acids and derivatives |
|---|
| Direct Parent | Hexacarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexacarboxylic acid or derivatives
- Biopterin
- Glutamic acid or derivatives
- Alpha-amino acid ester
- Pterin
- Alpha-amino acid or derivatives
- Pteridine
- Tertiary aliphatic/aromatic amine
- Amino fatty acid
- Aminopyrimidine
- Fatty acid ester
- Secondary aliphatic/aromatic amine
- Fatty acyl
- Pyrimidine
- Imidolactam
- Heteroaromatic compound
- Tertiary amine
- Amino acid or derivatives
- Amino acid
- Carboxylic acid ester
- Organoheterocyclic compound
- Secondary amine
- Azacycle
- Carboxylic acid
- Enamine
- Organic oxygen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Organic oxide
- Organopnictogen compound
- Primary amine
- Organic nitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|