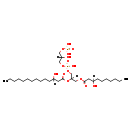| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 19:31:31 -0600 |
|---|
| Update Date | 2015-12-09 17:18:04 -0700 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | PGP(10:0(3-OH)/14:0(3-OH)) |
|---|
| Description | PGP(10:0(3-OH)/14:0(3-OH)) belongs to the class of glycerophosphoglycerophosphates, also called phosphatidylglycerophosphates (PGPs). These lipids contain a common glycerophosphate skeleton linked to at least one fatty acyl chain and a glycero-3-phosphate moiety. As is the case with diacylglycerols, phosphatidylglycerophosphates can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PGP(10:0(3-OH)/14:0(3-OH)), in particular, consists of one 3-hydroxydecanoyl chain to the C-1 atom, and one 3-hydroxytetradecanoyl to the C-2 atom. In E. coli, PGPs can be found in the cytoplasmic membrane. The are synthesized by the addition of glycerol 3-phosphate to a CDP-diacylglycerol. In turn, PGPs are dephosphorylated to Phosphatidylglycerols (PGs) by the enzyme Phosphatidylglycerophosphatase. |
|---|
| Structure | |
|---|
| Synonyms: | Not Available |
|---|
| Chemical Formula: | C30H60O15P2 |
|---|
| Weight: | Average: 722.743
Monoisotopic: 722.340745228 |
|---|
| InChI Key: | LUWQFGSTCVSUOG-XQOSCOKLSA-N |
|---|
| InChI: | InChI=1S/C30H60O15P2/c1-3-5-7-9-10-11-12-14-16-18-26(32)20-30(35)45-28(23-41-29(34)19-25(31)17-15-13-8-6-4-2)24-44-47(39,40)43-22-27(33)21-42-46(36,37)38/h25-28,31-33H,3-24H2,1-2H3,(H,39,40)(H2,36,37,38)/t25?,26?,27-,28-/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | [(2R)-2-hydroxy-3-({hydroxy[(2R)-3-[(3-hydroxydecanoyl)oxy]-2-[(3-hydroxytetradecanoyl)oxy]propoxy]phosphoryl}oxy)propoxy]phosphonic acid |
|---|
| Traditional IUPAC Name: | (2R)-2-hydroxy-3-{[hydroxy((2R)-3-[(3-hydroxydecanoyl)oxy]-2-[(3-hydroxytetradecanoyl)oxy]propoxy)phosphoryl]oxy}propoxyphosphonic acid |
|---|
| SMILES: | [H][C@@](O)(COP(O)(O)=O)COP(O)(=O)OC[C@@]([H])(COC(=O)CC(O)CCCCCCC)OC(=O)CC(O)CCCCCCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylglycerophosphates. These are glycerophosphoglycerophosphates in which two fatty acids are bonded to the 1-glycerol moiety through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoglycerophosphates |
|---|
| Direct Parent | Phosphatidylglycerophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycerophosphoglycerophosphate
- Sn-glycerol-3-phosphate
- Beta-hydroxy acid
- Fatty acid ester
- Dialkyl phosphate
- Monoalkyl phosphate
- Dicarboxylic acid or derivatives
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Hydroxy acid
- Organic phosphoric acid derivative
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -3 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | phospholipid biosynthesis (CL(16:1(9Z)/10:0/14:0/14:0)) | PW001954 |    |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|