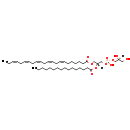| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 18:59:30 -0600 |
|---|
| Update Date | 2015-12-09 12:07:37 -0700 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | PG(16:0/22:5(7Z,10Z,13Z,16Z,19Z)) |
|---|
| Description | PG(16:0/22:5(7Z,10Z,13Z,16Z,19Z)) is a phosphatidylglycerol. Phosphatidylglycerols consist of a glycerol 3-phosphate backbone esterified to either saturated or unsaturated fatty acids on carbons 1 and 2. As is the case with diacylglycerols, phosphatidylglycerols can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PG(16:0/22:5(7Z,10Z,13Z,16Z,19Z)), in particular, consists of one hexadecanoyl chain to the C-1 atom, and one 7Z,10Z,13Z,16Z,19Z-docosapentaenoyl to the C-2 atom. In E. coli glycerophospholipid metabolism, phosphatidylglycerol is formed from phosphatidic acid (1,2-diacyl-sn-glycerol 3-phosphate) by a sequence of enzymatic reactions that proceeds via two intermediates, cytidine diphosphate diacylglycerol (CDP-diacylglycerol) and phosphatidylglycerophosphate (PGP, a phosphorylated phosphatidylglycerol). Phosphatidylglycerols, along with CDP-diacylglycerol, also serve as precursor molecules for the synthesis of cardiolipin, a phospholipid found in membranes. |
|---|
| Structure | |
|---|
| Synonyms: | | Value | Source |
|---|
| 1-clupanodonoyl-2-palmitoyl-sn-glycero-3-phospho-(1'-glycerol) | SMPDB, HMDB | | 1-clupanodonoyl-2-palmitoyl-sn-glycero-3-phosphoglycerol | SMPDB, HMDB | | PG(22:5/16:0) | SMPDB, HMDB | | PG(22:5n3/16:0) | SMPDB, HMDB | | PG(22:5w3/16:0) | SMPDB, HMDB | | PG(38:5) | SMPDB, HMDB | | Phosphatidylglycerol(22:5(7Z,10Z,13Z,16Z,19Z)/16:0) | SMPDB, HMDB | | Phosphatidylglycerol(22:5/16:0) | SMPDB, HMDB | | Phosphatidylglycerol(22:5n3/16:0) | SMPDB, HMDB | | Phosphatidylglycerol(22:5w3/16:0) | SMPDB, HMDB | | Phosphatidylglycerol(38:5) | SMPDB, HMDB | | PG(22:5(7Z,10Z,13Z,16Z,19Z)/16:0) | SMPDB | | PG(38:5) | Lipid Annotator | | PG(22:5/16:0) | Lipid Annotator | | 1-docosapentaenoyl-2-palmitoyl-sn-glycero-3-phosphoglycerol | Lipid Annotator, HMDB | | GPG(38:5) | Lipid Annotator, HMDB | | 1-(7Z,10Z,13Z,16Z,19Z-docosapentaenoyl)-2-hexadecanoyl-sn-glycero-3-phospho-(1'-glycerol) | Lipid Annotator, HMDB | | GPG(22:5/16:0) | Lipid Annotator, HMDB | | Phosphatidylglycerol(22:5/16:0) | Lipid Annotator | | 1-(7Z,10Z,13Z,16Z,19Z-docosapentaenoyl)-2-hexadecanoyl-sn-glycero-3-phosphoglycerol | Lipid Annotator, HMDB |
|
|---|
| Chemical Formula: | C44H77O10P |
|---|
| Weight: | Average: 797.064
Monoisotopic: 796.525435677 |
|---|
| InChI Key: | IUTIUWYTMFXMMO-RUVFBLPJSA-N |
|---|
| InChI: | InChI=1S/C44H77O10P/c1-3-5-7-9-11-13-15-17-18-19-20-21-22-24-25-27-29-31-33-35-43(47)51-39-42(40-53-55(49,50)52-38-41(46)37-45)54-44(48)36-34-32-30-28-26-23-16-14-12-10-8-6-4-2/h5,7,11,13,17-18,20-21,24-25,41-42,45-46H,3-4,6,8-10,12,14-16,19,22-23,26-40H2,1-2H3,(H,49,50)/b7-5-,13-11-,18-17-,21-20-,25-24-/t41-,42+/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | [(2S)-2,3-dihydroxypropoxy][(2R)-3-[(7Z,10Z,13Z,16Z,19Z)-docosa-7,10,13,16,19-pentaenoyloxy]-2-(hexadecanoyloxy)propoxy]phosphinic acid |
|---|
| Traditional IUPAC Name: | (2S)-2,3-dihydroxypropoxy((2R)-3-[(7Z,10Z,13Z,16Z,19Z)-docosa-7,10,13,16,19-pentaenoyloxy]-2-(hexadecanoyloxy)propoxy)phosphinic acid |
|---|
| SMILES: | [H][C@](O)(CO)COP(O)(=O)OC[C@@]([H])(COC(=O)CCCCC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC)OC(=O)CCCCCCCCCCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylglycerols. These are glycerophosphoglycerols in which two fatty acids are bonded to the 1-glycerol moiety through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoglycerols |
|---|
| Direct Parent | Phosphatidylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-diacylglycerophosphoglycerol
- Fatty acid ester
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- 1,2-diol
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Organic oxide
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Primary alcohol
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-4175352900-544e48d51ad60f24d74a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0570-5294221400-e4d5880f24993ee3c6b8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0c2i-9265112000-411f8a8d3cbcfbcf09b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-06vi-1149120500-b6417cd7a9e601fb7efa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-6229100100-877a02530cdd2509ad26 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9011000000-aac0a3cb9c2fc22eb72f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000000900-9869f21529424c0a4adf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6s-0179320700-8908ba5b067a32ea58f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a92-0279320700-bea8cb3cea623e00c933 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Uniprot Consortium (2012). "Reorganizing the protein space at the Universal Protein Resource (UniProt)." Nucleic Acids Res 40:D71-D75. Pubmed: 22102590
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB0116621 | | Pubchem Compound ID | 131823258 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|