| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:49:55 -0600 |
|---|
| Update Date | 2015-09-14 16:46:29 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
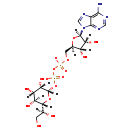
| Name: | ADP-L-glycero-beta-D-manno-heptose |
|---|
| Description | ADP-L-glycero-beta-D-manno-heptose is an intermediate in pathway Lipid A-core biosynthesis in E. coli. It is a substrate for enzymes ADP-heptose:Kdo2-lipid A heptosyltransferase, ADP-heptose:LPS heptosyltransferase, lipopolysaccharide core heptosyltransferase and lipid A-core heptosyltransferase. In pathway ADP-L-glycero-beta-D-manno-heptose biosynthesis, it is a product for enzyme ADP-L-glycero-D-mannoheptose-6-epimerase which catalyzes the reaction ADP-D-glycero-beta-D-manno-heptose -> ADP-L-glycero-beta-D-manno-heptose (PMID: 11751812; BioCyc compound: ADP-L-GLYCERO-D-MANNO-HEPTOSE). |
|---|
| Structure | |
|---|
| Synonyms: | - ADP-L-glycero-b-D-manno-Heptose
- ADP-L-glycero-β-D-manno-Heptose
|
|---|
| Chemical Formula: | C17H25N5O16P2 |
|---|
| Weight: | Average: 617.355
Monoisotopic: 617.078250901 |
|---|
| InChI Key: | KMSFWBYFWSKGGR-DTBZDYEHSA-L |
|---|
| InChI: | InChI=1S/C17H27N5O16P2/c18-14-7-15(20-3-19-14)22(4-21-7)16-11(28)8(25)6(35-16)2-34-39(30,31)38-40(32,33)37-17-12(29)9(26)10(27)13(36-17)5(24)1-23/h3-6,8-13,16-17,23-29H,1-2H2,(H,30,31)(H,32,33)(H2,18,19,20)/p-2/t5-,6+,8+,9-,10-,11+,12-,13+,16+,17-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl {[(2S,3S,4S,5S,6R)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl phosphonato]oxy}phosphonate |
|---|
| Traditional IUPAC Name: | [(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl [(2S,3S,4S,5S,6R)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl phosphonato]oxyphosphonate |
|---|
| SMILES: | [H][C@](O)(CO)[C@@]1([H])O[C@@]([H])(OP([O-])(=O)OP([O-])(=O)OC[C@@]2([H])O[C@@]([H])(N3C=NC4=C(N)N=CN=C34)[C@]([H])(O)[C@]2([H])O)[C@@]([H])(O)[C@@]([H])(O)[C@]1([H])O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as purine nucleotide sugars. These are purine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleotides |
|---|
| Sub Class | Purine nucleotide sugars |
|---|
| Direct Parent | Purine nucleotide sugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine nucleotide sugar
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Tetrahydrofuran
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- Polyol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Organic oxide
- Organic oxygen compound
- Alcohol
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Organonitrogen compound
- Primary alcohol
- Primary amine
- Organooxygen compound
- Organopnictogen compound
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | ADP-L-glycero-Beta-D-manno-heptose biosynthesis | PW002095 |    | | Lipopolysaccharide biosynthesis | PW000831 |    | | lipopolysaccharide biosynthesis II | PW001905 |    | | lipopolysaccharide biosynthesis III | PW002059 |    |
|
|---|
| KEGG Pathways: | - Lipopolysaccharide biosynthesis ec00540
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 61506 | | HMDB ID | Not Available | | Pubchem Compound ID | 46173344 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|