| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:22:38 -0600 |
|---|
| Update Date | 2015-10-15 16:14:24 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
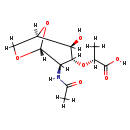
| Name: | 1,6-Anhydro-N-acetyl-beta-muramate |
|---|
| Description | 1,6-Anhydro-N-acetyl-beta-muramate monocarboxylic a obtained by removal of a proton from the carboxy group of 1,6-anhydro-N-acetyl-beta-muramic acid. It is a substrate of the enzyme anhydro-N-acetylmuramic acid kinase. The reaction it catalyzes is: ATP + 1,6-anhydro-N-acetyl-beta-muramate + H2O = ADP + N-acetylmuramate 6-phosphate. This enzyme, along with EC 4.2.1.126 (N-acetylmuramic acid 6-phosphate etherase) is required for the utilization of anhydro-N-acetylmuramic acid in proteobacteria. The substrate is either imported from the medium or derived from the bacterium's own cell wall murein during cell wall recycling. The product N-acetylmuramate 6-phosphate is produced as a 7:1 mixture of the α- and β-anomers. |
|---|
| Structure | |
|---|
| Synonyms: | - (2R)-2-(1R,2S,3R,4R,5R)-4-acetamido-2-Hydroxy-6,8-dioxabicyclo3.2.1octan-3-yloxypropanoate
- (2R)-2-(1R,2S,3R,4R,5R)-4-acetamido-2-hydroxy-6,8-dioxabicyclo3.2.1octan-3-yloxypropanoic acid
- (2R)-2-{(1R,2S,3R,4R,5R)-4-(acetylamino)-2-hydroxy-6,8-dioxabicyclo3.2.1oct-3-yloxy}propanoate
- (2R)-2-{(1R,2S,3R,4R,5R)-4-(acetylamino)-2-hydroxy-6,8-dioxabicyclo3.2.1oct-3-yloxy}propanoic acid
- (2R)-2-{(1R,2S,3R,4R,5R)-4-acetamido-2-hydroxy-6,8-dioxabicyclo3.2.1oct-3-yloxy}propanoate (non-preferred name)
- (2R)-2-{(1R,2S,3R,4R,5R)-4-Acetamido-2-hydroxy-6,8-dioxabicyclo3.2.1oct-3-yloxy}propanoic acid (non-preferred name)
- 1,6-anhMurNAc
- 1,6-anhydro-N-Acetyl-b-muramate
- 1,6-anhydro-N-Acetyl-b-muramic acid
- 1,6-anhydro-N-Acetyl-beta-muramic acid
- 1,6-anhydro-N-Acetyl-muramate
- 1,6-anhydro-N-acetyl-muramic acid
- 1,6-anhydro-N-Acetyl-β-muramate
- 1,6-anhydro-N-Acetyl-β-muramic acid
- 1,6-anhydro-N-Acetylmuramate
- 1,6-anhydro-N-acetylmuramic acid
- 2-(2-acetylamino-4-HYDROXY-6,8-dioxa-bicyclo3.2.1oct-3-yloxy)-propionate
- 2-(2-ACETYLAMINO-4-HYDROXY-6,8-DIOXA-BICYCLO3.2.1OCT-3-YLOXY)-PROPIONIC ACID
|
|---|
| Chemical Formula: | C11H16NO7 |
|---|
| Weight: | Average: 274.2472
Monoisotopic: 274.092676871 |
|---|
| InChI Key: | ZFEGYUMHFZOYIY-YVNCZSHWSA-M |
|---|
| InChI: | InChI=1S/C11H17NO7/c1-4(10(15)16)18-9-7(12-5(2)13)11-17-3-6(19-11)8(9)14/h4,6-9,11,14H,3H2,1-2H3,(H,12,13)(H,15,16)/p-1/t4-,6-,7-,8-,9-,11-/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2R)-2-{[(1R,2S,3R,4R,5R)-4-acetamido-2-hydroxy-6,8-dioxabicyclo[3.2.1]octan-3-yl]oxy}propanoic acid |
|---|
| Traditional IUPAC Name: | 1,6-anhMurNAc |
|---|
| SMILES: | C[C@@H](O[C@H]1[C@H](O)[C@H]2CO[C@H](O2)[C@@H]1N=C(C)[O-])C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oxepanes. Oxepanes are compounds containing an oxepane ring, which is a seven-member saturated aliphatic heterocycle with one oxygen and six carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Oxepanes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Oxepanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxepane
- Monosaccharide
- Oxane
- Meta-dioxolane
- Acetamide
- Carboxamide group
- Secondary alcohol
- Secondary carboxylic acid amide
- Acetal
- Carboxylic acid derivative
- Carboxylic acid
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Carbonyl group
- Alcohol
- Organic oxygen compound
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 40666 | | HMDB ID | Not Available | | Pubchem Compound ID | 6602346 | | Kegg ID | C19769 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available | | Ligand Expo | AH0 |
|
|---|