| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:18:24 -0600 |
|---|
| Update Date | 2015-09-16 14:50:11 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Geranyl diphosphate |
|---|
| Description | Geranyl diphosphate is regarded as a key intermediate in the steroid, isoprene and terpene biosynthesis pathways and is used in the biosynthesis of farnesyl pyrophosphate, geranylgeranyl pyrophosphate, cholesterol, terpenes and terpenoids. (wikipedia).
|
|---|
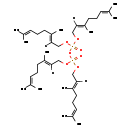
| Structure | |
|---|
| Synonyms: | - ω,E-geranyl diphosphate
- ω,e-geranyl diphosphoric acid
- Geranyl diphosphoric acid
- Geranyl pyrophosphate
- Geranyl pyrophosphoric acid
- Geranyl-diphosphate
- Geranyl-diphosphoric acid
- Geranyl-PP
- Geranyl-pyrophosphate
- Geranyl-pyrophosphoric acid
- GPP
|
|---|
| Chemical Formula: | C40H68O7P2 |
|---|
| Weight: | Average: 722.9112
Monoisotopic: 722.444027554 |
|---|
| InChI Key: | CNCHONBZLLTYBW-NISPWTRESA-N |
|---|
| InChI: | InChI=1S/C40H68O7P2/c1-33(2)17-13-21-37(9)25-29-43-48(41,44-30-26-38(10)22-14-18-34(3)4)47-49(42,45-31-27-39(11)23-15-19-35(5)6)46-32-28-40(12)24-16-20-36(7)8/h17-20,25-28H,13-16,21-24,29-32H2,1-12H3/b37-25+,38-26+,39-27+,40-28+ |
|---|
| CAS number: | 763-10-0 |
|---|
| IUPAC Name: | bis(2E)-3,7-dimethylocta-2,6-dien-1-yl {[bis({[(2E)-3,7-dimethylocta-2,6-dien-1-yl]oxy})phosphoryl]oxy}phosphonate |
|---|
| Traditional IUPAC Name: | bis(2E)-3,7-dimethylocta-2,6-dien-1-yl {bis[(2E)-3,7-dimethylocta-2,6-dien-1-yl]oxyphosphoryl}oxyphosphonate |
|---|
| SMILES: | [H]\C(COP(=O)(OC\C([H])=C(/C)CCC=C(C)C)OP(=O)(OC\C([H])=C(/C)CCC=C(C)C)OC\C([H])=C(/C)CCC=C(C)C)=C(\C)CCC=C(C)C |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isoprenoid phosphates. These are prenol lipids containing a phosphate group linked to an isoprene (2-methylbuta-1,3-diene) unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Isoprenoid phosphates |
|---|
| Direct Parent | Isoprenoid phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic pyrophosphate
- Monoterpenoid
- Isoprenoid phosphate
- Acyclic monoterpenoid
- Dialkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|