| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:15:16 -0600 |
|---|
| Update Date | 2015-09-17 15:42:01 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | 2-Dehydro-3-deoxy-D-galactonate 6-phosphate |
|---|
| Description | 2-Dehydro-3-deoxy-D-galactonate-6-phosphate is a member of the chemical class known as Organophosphate Esters. These are organic compounds containing phosphoric acid ester functional group. It is a substrate for 2-dehydro-3-deoxy-6-phosphogalactonate aldolase which breaks it down to pyruvate and D-glyceraldehyde 3-phosphate. It is a product of carbohydrate acid metabolism, specifically D-galactonate degradation. |
|---|
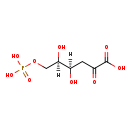
| Structure | |
|---|
| Synonyms: | - (4R,5R)-4,5-Dihydroxy-2-oxo-6-phosphonooxyhexanoate
- (4R,5R)-4,5-dihydroxy-2-oxo-6-phosphonooxyhexanoic acid
- 2-dehydro-3-deoxy-D-galactonate 6-phosphate
- 2-dehydro-3-Deoxy-D-galactonic acid 6-phosphoric acid
- 3-Deoxy-6-O-phosphono-D-threo-hex-2-ulosonate
- 3-Deoxy-6-O-phosphono-D-threo-hex-2-ulosonic acid
|
|---|
| Chemical Formula: | C6H8O9P |
|---|
| Weight: | Average: 255.096
Monoisotopic: 254.992239575 |
|---|
| InChI Key: | OVPRPPOVAXRCED-UHFFFAOYSA-K |
|---|
| InChI: | InChI=1S/C6H11O9P/c7-3(1-4(8)6(10)11)5(9)2-15-16(12,13)14/h3,5,7,9H,1-2H2,(H,10,11)(H2,12,13,14)/p-3 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (4R,5R)-4,5-dihydroxy-2-oxo-6-(phosphonooxy)hexanoic acid |
|---|
| Traditional IUPAC Name: | (4R,5R)-4,5-dihydroxy-2-oxo-6-(phosphonooxy)hexanoic acid |
|---|
| SMILES: | OC(COP([O-])([O-])=O)C(O)CC(=O)C([O-])=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain keto acids and derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Keto acids and derivatives |
|---|
| Sub Class | Medium-chain keto acids and derivatives |
|---|
| Direct Parent | Medium-chain keto acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain keto acid
- Monoalkyl phosphate
- Alpha-keto acid
- Beta-hydroxy ketone
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Alpha-hydroxy ketone
- Ketone
- 1,2-diol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -3 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Galactitol and galactonate degradation | PW000820 |    |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 17860 | | HMDB ID | Not Available | | Pubchem Compound ID | 5459949 | | Kegg ID | C01286 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available | | Ligand Expo | KDP |
|
|---|