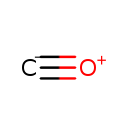Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
CO (M2MDB003394)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-10-10 12:11:55 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-10-02 02:25:50 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | CO | ||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Carbon monoxide, with the chemical formula CO, is a colorless, odorless, and tasteless gas. It consists of one carbon atom covalently bonded to one oxygen atom. It is a gas at room temperature. Carbon monoxide is an intermediate in 4-amino-2-methyl-5-diphosphomethylpyrimidine biosynthesis in E. coli. It is a product for enzyme HMP-P synthase which catalyzes the chemical reaction 5-amino-1-(5-phospho-beta-D-ribosyl)imidazole + S-adenosyl-L-methionine -> 4-amino-2-methyl-5-phosphomethylpyrimidine + 5'-deoxyadenosine + L-methionine + formate + carbon monoxide + 3 H+ (EcoCyc compound: CARBON-MONOXIDE). | ||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | CO | ||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 28.01 Monoisotopic: 27.99491462 | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | UGFAIRIUMAVXCW-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/CO/c1-2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 124-38-9 | ||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of inorganic compounds known as homogeneous other non-metal compounds. These are inorganic non-metallic compounds in which the largest atom belongs to the class of 'other non-metals'. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Homogeneous other non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Homogeneous other non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | -56.5 C | ||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 4-Amino-5-hydroxymethyl-2-methylpyrimidine + S-Adenosylmethionine <> 5-Aminoimidazole ribonucleotide + 4-Amino-2-methyl-5-phosphomethylpyrimidine + 5'-Deoxyadenosine + L-Methionine + Formic acid + CO 5-Aminoimidazole ribonucleotide + S-adenosyl-L-methionine > 4-Amino-2-methyl-5-phosphomethylpyrimidine + 5'-Deoxyadenosine + L-Methionine + Formic acid + CO Quercetin + Oxygen > 2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoate + CO + Hydrogen ion 5-Aminoimidazole ribonucleotide + S-adenosyl-L-methionine >3 Hydrogen ion + CO + Formic acid + L-Methionine + 5'-Deoxyadenosine + 4-amino-2-methyl-5-phosphomethylpyrimidine 4 4-Amino-5-hydroxymethyl-2-methylpyrimidine + S-Adenosylmethionine <>5 5-Aminoimidazole ribonucleotide +4 4-Amino-2-methyl-5-phosphomethylpyrimidine +5 5'-Deoxyadenosine + L-Methionine + Formic acid + CO 4 4-Amino-5-hydroxymethyl-2-methylpyrimidine + S-Adenosylmethionine <>5 5-Aminoimidazole ribonucleotide +4 4-Amino-2-methyl-5-phosphomethylpyrimidine +5 5'-Deoxyadenosine + L-Methionine + Formic acid + CO | ||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||
| References: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Ivanova, Svetlana; Pitchon, Veronique; Petit, Corinne. Application of the direct exchange method in the preparation of gold catalysts supported on different oxide materials. Journal of Molecular Catalysis A: Chemical (2006), 256(1-2), 278-283. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in thiamine biosynthetic process
- Specific function:
- Catalyzes the synthesis of the hydroxymethylpyrimidine phosphate (HMP-P) moiety of thiamine from aminoimidazole ribotide (AIR) in a radical S-adenosyl-L-methionine (SAM)-dependent reaction
- Gene Name:
- thiC
- Uniprot ID:
- P30136
- Molecular weight:
- 70850
Reactions
| 5-amino-1-(5-phospho-D-ribosyl)imidazole + S-adenosyl-L-methionine = 4-amino-2-methyl-5-phosphomethylpyrimidine + 5'-deoxyadenosine + L-methionine + formate + CO. |