| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-15 08:51:33 -0600 |
|---|
| Update Date | 2015-09-13 12:56:17 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Galactan |
|---|
| Description | Galactan is a beta-glucan consisting of polymerized galactose. Beta -glucans are glucose polymers found in the cell walls of plants, fungi, and bacteria and as conserved structures can be considered to be classical pathogen-associated molecular patterns. |
|---|
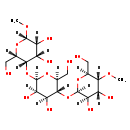
| Structure | |
|---|
| Synonyms: | |
|---|
| Chemical Formula: | C20H36O16 |
|---|
| Weight: | Average: 532.4902
Monoisotopic: 532.200335104 |
|---|
| InChI Key: | HYRUXGRHTJRKNG-IESUNRGTSA-N |
|---|
| InChI: | InChI=1S/C20H36O16/c1-30-15-6(3-21)33-19(13(28)9(15)24)36-17-8(5-23)34-20(14(29)11(17)26)35-16-7(4-22)32-18(31-2)12(27)10(16)25/h6-29H,3-5H2,1-2H3/t6-,7-,8-,9+,10-,11-,12+,13+,14+,15-,16+,17-,18-,19+,20+/m0/s1 |
|---|
| CAS number: | 39300-87-3 |
|---|
| IUPAC Name: | (2S,3R,4S,5S,6S)-5-{[(2R,3R,4S,5R,6S)-5-{[(2R,3R,4R,5R,6S)-3,4-dihydroxy-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-(hydroxymethyl)-2-methoxyoxane-3,4-diol |
|---|
| Traditional IUPAC Name: | (2S,3R,4S,5S,6S)-5-{[(2R,3R,4S,5R,6S)-5-{[(2R,3R,4R,5R,6S)-3,4-dihydroxy-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-(hydroxymethyl)-2-methoxyoxane-3,4-diol |
|---|
| SMILES: | [H][C@@]1(O)[C@]([H])(O)[C@]([H])(O[C@@]2([H])O[C@@]([H])(CO)[C@]([H])(O[C@@]3([H])O[C@@]([H])(CO)[C@]([H])(OC)[C@]([H])(O)[C@@]3([H])O)[C@@]([H])(O)[C@@]2([H])O)[C@]([H])(CO)O[C@]1([H])OC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- O-glycosyl compound
- Glycosyl compound
- Oxane
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Ether
- Dialkyl ether
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| Links |
|---|
| External Links: | |
|---|