| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-09 09:25:25 -0600 |
|---|
| Update Date | 2015-06-03 17:21:46 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Menaquinol 6 |
|---|
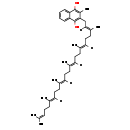
| Description | Menaquinol 6 is a polyprenylhydroquinone having a hexaprenyl moiety at position 2 and a methyl group at position 3. It is a substrate for Dimethyl sulfoxide reductase (dmsA). This enzyme catalyzes the reduction of dimethyl sulfoxide (DMSO) to dimethyl sulfide (DMS) using the following reaction: Dimethylsulfide + menaquinone + H2O = dimethylsulfoxide + menaquinol. DMSO reductase serves as the terminal reductase under anaerobic conditions, with DMSO being the terminal electron acceptor. Terminal reductase during anaerobic growth on various sulfoxides and N-oxide compounds. This enzyme allows E.coli to grow anaerobically on DMSO as respiratory oxidant. Menaquinol 6 is generated by Ubiquinone/menaquinone biosynthesis methyltransferase (ubiE). This enzyme is required for the conversion of demethylmenaquinone (DMKH2) to menaquinone (MKH2) and has the following catalytic activity: A demethylmenaquinone + S-adenosyl-L-methionine = a menaquinol + S-adenosyl-L-homocysteine. |
|---|
| Structure | |
|---|
| Synonyms: | - A Menaquinol
- Menaquinol
- Reduced menaquinone
- Reduced vitamin K2
- Vitamin K2 hydroquinone
|
|---|
| Chemical Formula: | C41H58O2 |
|---|
| Weight: | Average: 582.898
Monoisotopic: 582.4436811 |
|---|
| InChI Key: | ZVENTDGZQVBWNA-RCIYGOBDSA-N |
|---|
| InChI: | InChI=1S/C41H58O2/c1-30(2)16-11-17-31(3)18-12-19-32(4)20-13-21-33(5)22-14-23-34(6)24-15-25-35(7)28-29-37-36(8)40(42)38-26-9-10-27-39(38)41(37)43/h9-10,16,18,20,22,24,26-28,42-43H,11-15,17,19,21,23,25,29H2,1-8H3/b31-18+,32-20+,33-22+,34-24+,35-28+ |
|---|
| CAS number: | 39776-48-2 |
|---|
| IUPAC Name: | 2-[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]-3-methylnaphthalene-1,4-diol |
|---|
| Traditional IUPAC Name: | 2-[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]-3-methylnaphthalene-1,4-diol |
|---|
| SMILES: | [H]\C(CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC1=C(O)C2=CC=CC=C2C(O)=C1C)=C(\C)CCC=C(C)C |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as polyprenylphenols. Polyprenylphenols are compounds containing a polyisoprene chain attached to a phenol group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Polyprenylphenols |
|---|
| Direct Parent | Polyprenylphenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polyprenylphenol
- Sesterterpenoid
- Prenylbenzoquinol
- 1-naphthol
- Naphthalene
- Hydroquinone
- Benzenoid
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | - Metabolic pathways eco01100
- Porphyrin and chlorophyll metabolism ec00860
- Sulfur metabolism ec00920
- Ubiquinone and other terpenoid-quinone biosynthesis ec00130
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 18151 | | HMDB ID | Not Available | | Pubchem Compound ID | 6441040 | | Kegg ID | C05819 | | ChemSpider ID | 4945264 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|