| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-09 09:25:23 -0600 |
|---|
| Update Date | 2015-09-13 12:56:17 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
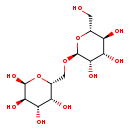
| Name: | Isomaltose |
|---|
| Description | Isomaltose is a naturally occurring disaccharide of two glucose units similar to maltose, but with a alpha-(1-6)-linkage instead of the alpha-(1-4)-linkage. It is a reducing sugar. Both of the sugars are glucose and pyranoses. Isomaltose is produced when high maltose syrup is treated with the enzyme transglucosidase (TG) and is one of the major components in the mixture isomaltooligosaccharide. |
|---|
| Structure | |
|---|
| Synonyms: | - 6-O-a-D-Glucopyranosyl-D-glucose
- 6-O-a-delta-Glucopyranosyl-delta-glucose
- 6-O-a-δ-Glucopyranosyl-δ-glucose
- 6-O-alpha-D-Glucopyranosyl-D-glucose
- 6-O-alpha-delta-Glucopyranosyl-delta-glucose
- 6-O-α-D-Glucopyranosyl-D-glucose
- 6-O-α-δ-Glucopyranosyl-δ-glucose
- a-D-Glucopyranosyl-(1Right6)-D-glucose
- Alpha-D-Glucopyranosyl-(1right6)-D-glucose
- Brachiose
- Isomaltose
- α-D-Glucopyranosyl-(1Right6)-D-glucose
|
|---|
| Chemical Formula: | C12H22O11 |
|---|
| Weight: | Average: 342.2965
Monoisotopic: 342.116211546 |
|---|
| InChI Key: | DLRVVLDZNNYCBX-RTPHMHGBSA-N |
|---|
| InChI: | InChI=1S/C12H22O11/c13-1-3-5(14)8(17)10(19)12(23-3)21-2-4-6(15)7(16)9(18)11(20)22-4/h3-20H,1-2H2/t3-,4-,5-,6-,7+,8+,9-,10-,11?,12+/m1/s1 |
|---|
| CAS number: | 499-40-1 |
|---|
| IUPAC Name: | (2S,3R,4S,5R,6R)-6-({[(2S,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxane-2,3,4,5-tetrol |
|---|
| Traditional IUPAC Name: | epimelibiose |
|---|
| SMILES: | OC[C@H]1O[C@H](OC[C@H]2OC(O)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@@H](O)[C@@H]1O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-glycosyl compound
- Disaccharide
- Oxane
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (8 TMS) | splash10-0uxr-0941000000-58f859df76fe1e87b22a | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (8 TMS) | splash10-0udi-0941000000-58d4fa7d40972e66a89d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0wmj-0951000000-0023b7689b93195cc520 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0uxr-0941000000-1bcc10f21d6677bb0060 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (8 TMS; 1 MEOX) | splash10-00di-9741000000-a0071ea1c00218406733 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (8 TMS; 1 MEOX) | splash10-00di-9841000000-2028da966c304a1cd6cf | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0uxr-0941000000-58f859df76fe1e87b22a | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0udi-0941000000-58d4fa7d40972e66a89d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0wmj-0951000000-0023b7689b93195cc520 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0uxr-0941000000-1bcc10f21d6677bb0060 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9741000000-a0071ea1c00218406733 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9841000000-2028da966c304a1cd6cf | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0wmj-0941000000-a42b8ddd325c04e1ac1a | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0uxr-0941000000-e12e0f47b8ab7a54784e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0iu0-2695000000-b644ad8ee930f0244c0f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-014i-5652219000-e37450d07ee330d4fac3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-01ox-1709000000-e1aedea040f248898825 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-9501000000-dfecff3e8858cc005d12 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-000i-9000000000-1e5c79ae513f06c8d563 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0309000000-5545d2826c364f86c437 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-0902000000-842fab6069f2dd5af792 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ikd-6940000000-16b9872436436b7073ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1449000000-768cbc294d03684f91a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0229-5944000000-b042101ecaa99993cfe1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054o-9620000000-97071ace9e7da2591fd0 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Guddat S, Thevis M, Schanzer W: Identification and quantification of the plasma volume expander dextran in human urine by liquid chromatography-tandem mass spectrometry of enzymatically derived isomaltose. Biomed Chromatogr. 2005 Dec;19(10):743-50. Pubmed: 15856492
- Kuriyama M, Hiwatari R, Osame M, Igata A: Leucocyte alpha-1,4- and alpha-1,6-glucosidase activities towards oligosaccharides in late onset glycogenosis type II. Tohoku J Exp Med. 1990 Aug;161(4):343-51. Pubmed: 2256108
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Vitek V, Vitek K: Chromatography of sugars in body fluids. IV. Separation of isomaltose and lactose in urine by paper chromatography. Biochem Med. 1973 Feb;7(1):119-27. Pubmed: 4684081
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| Links |
|---|
| External Links: | |
|---|