| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-03 14:57:55 -0600 |
|---|
| Update Date | 2015-09-13 15:15:32 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Diethanolamine |
|---|
| Description | Diethanolamine, often abbreviated as DEA, is an organic chemical compound which is both a secondary amine and a dialcohol. A dialcohol has two hydroxyl groups in its molecule. Like other amines, diethanolamine acts as a weak base. Diethanolamine is widely used in the preparation of diethanolamides and diethanolamine salts of long-chain fatty acids that are formulated into soaps and surfactants used in liquid laundry and dishwashing detergents, cosmetics, shampoos, and hair conditioners. Diethanolamine is also used in textile processing, in industrial gas purification to remove acid gases, as an anticorrosion agent in metalworking fluids, and in preparations of agricultural chemicals. Aqueous diethanolamine solutions are used as solvents for numerous drugs that are administered intravenously. |
|---|
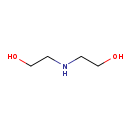
| Structure | |
|---|
| Synonyms: | - 2,2'-Dihydroxydiethylamine
- 2,2'-Iminobis
- 2,2'-Iminobisethanol
- 2,2'-Iminodi-1-ethanol
- 2,2'-Iminodiethanol
- 2,2'Iminobisethanol
- 2-(2-Hydroxyethylamino)ethanol
- 2-[(2-Hydroxyethyl)amino]ethanol
- Aliphatic amine
- B,b'-Dihydroxydiethylamine
- Bis(2-hydroxyethyl)amine
- Bis(2-hydroxyethyl)tallow amine oxide
- Bis(hydroxyethyl)amine
- Bis-2-hydroxyethylamine
- DEA
- DEOA
- Di(2-hydroxyethyl)amine
- Di(b-hydroxyethyl)amine
- Di(beta-hydroxyethyl)amine
- Di(β-hydroxyethyl)amine
- Diaethanolamin
- Diethanolamin
- Diethylolamine
- Dihydroxyethyl tallowamine oxide
- Diolamine
- H2dea
- Iminodiethanol
- N,N'-Iminodiethanol
- N,N-Bis(2-hydroxyethyl)amine
- N,N-Di(hydroxyethyl)amine
- N,N-Diethanolamine
- N-Ethylethanamine
- Niax DEOA-LF
|
|---|
| Chemical Formula: | C4H11NO2 |
|---|
| Weight: | Average: 105.1356
Monoisotopic: 105.078978601 |
|---|
| InChI Key: | ZBCBWPMODOFKDW-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H11NO2/c6-3-1-5-2-4-7/h5-7H,1-4H2 |
|---|
| CAS number: | 111-42-2 |
|---|
| IUPAC Name: | 2-[(2-hydroxyethyl)amino]ethan-1-ol |
|---|
| Traditional IUPAC Name: | diethanolamine |
|---|
| SMILES: | OCCNCCO |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-aminoalcohols. These are organic compounds containing an alkyl chain with an amine group bound to the C1 atom and an alcohol group bound to the C2 atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | 1,2-aminoalcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-aminoalcohol
- Secondary amine
- Secondary aliphatic amine
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 1 |
|---|
| Melting point: | 28 °C |
|---|
| Experimental Properties: | | Property | Value | Source |
|---|
| Water Solubility: | 1000 mg/mL at 20 oC [DOW CHEMICAL COMPANY (1980)] | PhysProp | | LogP: | -1.43 [HANSCH,C ET AL. (1995)] | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| | Concentration | Strain | Media | Growth Status | Growth System | Temperature | Details |
|---|
| 20± 0 uM | BW25113 | 48 mM Na2HPO4, 22 mM KH2PO4, 10 mM NaCl, 45 mM (NH4)2SO4, supplemented with 1 mM MgSO4, 1 mg/l thiamine·HCl, 5.6 mg/l CaCl2, 8 mg/l FeCl3, 1 mg/l MnCl2·4H2O, 1.7 mg/l ZnCl2, 0.43 mg/l CuCl2·2H2O, 0.6 mg/l CoCl2·2H2O and 0.6 mg/l Na2MoO4·2H2O. 4 g/L Gluco | Stationary Phase, glucose limited | Bioreactor, pH controlled, O2 and CO2 controlled, dilution rate: 0.2/h | 37 oC | PMID: 17379776 |
|
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-00di-9430000000-79f7d3e25a715793917a | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-001j-1900000000-bd0861145364bd684950 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-0159-5940000000-ebe5523dad23d3dc2391 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9430000000-79f7d3e25a715793917a | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0159-5940000000-ebe5523dad23d3dc2391 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-001j-1900000000-bd0861145364bd684950 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05am-9000000000-7eea9eaa21af51853910 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00dj-9800000000-3db6474ab842a5d03fb3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0a4r-9700000000-6ae8b510d6cd7e4ba8d0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0005-9000000000-d0e266aca011203c762a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0005-9000000000-b924f709a47552d0afc1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0a4i-0900000000-11bfee15df34ebc320e8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-05g0-9200000000-39da135b20d99fa34636 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-00dm-9000000000-74add71de850cb4c8e2d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0005-9000000000-794a61320b2e8c5a8970 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-0007-9000000000-af9eeb72884d4847a0a1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0a4i-0900000000-cd466740723ff131d76f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0a4i-0900000000-a5ca41984423de95ef78 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-05g0-9200000000-626e5d5103e3012c611e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-00dm-9000000000-74add71de850cb4c8e2d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0005-9000000000-794a61320b2e8c5a8970 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0007-9000000000-be35c09bcc6cd8c79f05 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0a4i-0900000000-cd466740723ff131d76f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0abi-9500000000-ee4dbde39d4c97f1cae9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-03k9-9000000000-e03c494989bc56f168ee | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9000000000-122a3a485bc0a1bfded4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9000000000-939cd379fb164bb8a094 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2900000000-c1dc229350e5cf19c090 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-9500000000-b3018e09980645272c12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9000000000-ae6bfcca013a433a0a24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-3900000000-d6387d7e1686e32ad49a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-8900000000-66c6a6cce5f90a2cdb52 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ox-9000000000-7e1da753c608954b27ee | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-05fr-9000000000-362b201708dabf55f01b | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Ishii, N., Nakahigashi, K., Baba, T., Robert, M., Soga, T., Kanai, A., Hirasawa, T., Naba, M., Hirai, K., Hoque, A., Ho, P. Y., Kakazu, Y., Sugawara, K., Igarashi, S., Harada, S., Masuda, T., Sugiyama, N., Togashi, T., Hasegawa, M., Takai, Y., Yugi, K., Arakawa, K., Iwata, N., Toya, Y., Nakayama, Y., Nishioka, T., Shimizu, K., Mori, H., Tomita, M. (2007). "Multiple high-throughput analyses monitor the response of E. coli to perturbations." Science 316:593-597. Pubmed: 17379776
- Mathews JM, deCosta K, Thomas BF: Lauramide diethanolamine absorption, metabolism, and disposition in rats and mice after oral, intravenous, and dermal administration. Drug Metab Dispos. 1996 Jul;24(7):702-10. Pubmed: 8818565
- NTP Toxicology and Carcinogenesis Studies of Diethanolamine (CAS No. 111-42-2) in F344/N Rats and B6C3F1 Mice (Dermal Studies). Natl Toxicol Program Tech Rep Ser. 1999 Jul;478:1-212. Pubmed: 12571685
- NTP Toxicology and Carcinogenesis Studies of Lauric Acid Diethanolamine Condensate (CAS NO. 120-40-1) in F344/N Rats and B6C3F1 Mice (Dermal Studies). Natl Toxicol Program Tech Rep Ser. 1999 Jul;480:1-200. Pubmed: 12571683
- Zeisel SH, DaCosta KA, Fox JG: Endogenous formation of dimethylamine. Biochem J. 1985 Dec 1;232(2):403-8. Pubmed: 4091797
|
|---|
| Synthesis Reference: | Peschel, Werner; Hildebrandt, Axel; Bessling, Bernd. Continuous process for the synthesis of monoethanolamine, diethanolamine and triethanolamine via the addition reaction of ammonia with ethylene oxide in the presence of water as a catalyst. Eur. Pat. Appl. (2003), 11 pp. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| Links |
|---|
| External Links: | |
|---|