| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:27:19 -0600 |
|---|
| Update Date | 2015-06-03 17:19:18 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
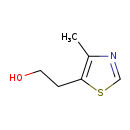
| Name: | 5-(2-Hydroxyethyl)-4-methylthiazole |
|---|
| Description | 5-(2-hydroxyethyl)-4-methylthiazole is a member of the chemical class known as Thiazoles. These are heterocyclic compounds containing a five-member aromatic ring made up of one sulfur atom, one nitrogen, and three carbon atoms. |
|---|
| Structure | |
|---|
| Synonyms: | - (2-hydroxyethyl)thiazole
- 2-(4-Methyl-1,3-thiazol-5-yl)ethanol
- 2-(4-METHYL-THIAZOL-5-YL)-ETHANOL
- 2-(4-Methylthiazol-5-yl)ethanol
- 2-(4-Methylthiazole-5-yl)ethanol
- 4-Methyl-5-(.beta.-hydroxyethyl)thiazole
- 4-Methyl-5-(2'-hydroxyethyl)-thiazole
- 4-Methyl-5-(2-hydroxyethyl)-thiazole
- 4-Methyl-5-(2-hydroxyethyl)thiazole
- 4-Methyl-5-(b-hydroxyethyl)thiazole
- 4-Methyl-5-(beta-hydroxyethyl)thiazole
- 4-Methyl-5-(β-hydroxyethyl)thiazole
- 4-Methyl-5-hydroxethylthiazole
- 4-Methyl-5-hydroxyethylthiazole
- 4-Methyl-5-thiazoleethanol
- 4-Methyl-5-thiazolethanol
- 4-Methyl-5-thiazolylethanol
- 5-(2-hydroxyethyl)-4-methylthiazole
- 5-(2-Hydroxyethyl)-4-methylthiazole (sulfurol)
- 5-(2-Hydroxyethyl)-4-methylthiazole (sulphurol)
- 5-(b-Hydroxyethyl)-4-methylthiazole
- 5-(beta-Hydroxyethyl)-4-methylthiazole
- 5-(Hydroxyethyl)-4-methylthiazole
- 5-(β-Hydroxyethyl)-4-methylthiazole
- Hemineurine
- HET
- MHT
- MHT (van)
- NChemBio.2007.13-comp11
- Sulfurol
- Sulphurol
- Thiamine breakdown product 4-methyl-5-thiazoleethanol- from
- Thiamine thiazole
- Thiazole, 5-(2-hydroxyethyl)-4-methyl
- THZ
- TZE
|
|---|
| Chemical Formula: | C6H9NOS |
|---|
| Weight: | Average: 143.207
Monoisotopic: 143.040484605 |
|---|
| InChI Key: | BKAWJIRCKVUVED-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C6H9NOS/c1-5-6(2-3-8)9-4-7-5/h4,8H,2-3H2,1H3 |
|---|
| CAS number: | 137-00-8 |
|---|
| IUPAC Name: | 2-(4-methyl-1,3-thiazol-5-yl)ethan-1-ol |
|---|
| Traditional IUPAC Name: | 4-methyl-5-thiazoleethanol |
|---|
| SMILES: | CC1=C(CCO)SC=N1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 4,5-disubstituted thiazoles. 4,5-disubstituted thiazoles are compounds containing a thiazole ring substituted at positions 4 and 5 only. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Thiazoles |
|---|
| Direct Parent | 4,5-disubstituted thiazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4,5-disubstituted 1,3-thiazole
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 0 |
|---|
| Melting point: | < 25 °C |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-0udi-3910000000-2c7da803c60f23e92cb8 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03di-3900000000-373fe84bca11e1b9a8e8 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0udi-3910000000-2c7da803c60f23e92cb8 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0udi-2910000000-917762a1db7938b1744a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-5900000000-a533361156b4169f3662 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-9320000000-2699a9d0d4585266a9fb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0006-0900000000-752807b0316740b2f094 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0006-0900000000-78f29564099d057faa39 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-03di-1900000000-eed8b0468f9906ba231c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-03di-3900000000-f44ad5d4a3225d9b0bcc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-03di-9700000000-33a95f67a6613491886d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-01ox-0900000000-06db10539c8be16d6109 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-03di-1900000000-0c0977714e57292d99f9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-03dl-0900000000-88e26c627663843f9708 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-03di-1900000000-0c0977714e57292d99f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0900000000-77f0167f1adcb7bd13de | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1900000000-7716ea7b3c8440649464 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ta-9500000000-67d0defea9e8825ae1aa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01ox-1900000000-11ac5d4965cdb773ab47 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006w-9600000000-fbed1a518b95bea3510c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-7872cc957fe09907a8b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0900000000-2e5c326d07e929018d95 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01u3-8900000000-c889afd6f3bb43d37fdb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9100000000-b0bb72c5777ad7b7b1a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9100000000-b34c758ceec8653ad03f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-9300000000-70a6aa386fb75b9d9b36 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-bb4dcf1719345df44077 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|