Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
2-Hydroxy-2,4-pentadienoate (M2MDB000890)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 14:24:21 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-10-15 16:14:56 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2-Hydroxy-2,4-pentadienoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 2-hydroxy-2,4-pentadienoate is a member of the chemical class known as Unsaturated Fatty Acids. These are fatty acids whose chain contains at least one CC double bond. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
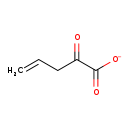
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C5H6O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 114.0993 Monoisotopic: 114.031694058 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | NOXRYJAWRSNUJD-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C5H6O3/c1-2-3-4(6)5(7)8/h2H,1,3H2,(H,7,8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 159694-16-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-oxopent-4-enoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | oxopent-4-enoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC(=O)C(=O)CC=C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Short-chain keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Short-chain keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Water + 2-Hydroxy-6-ketononadienedicarboxylate > Hydrogen ion + 2-Hydroxy-2,4-pentadienoate + Succinic acid Water + 2-Hydroxy-6-ketononatrienedioate > Fumaric acid + Hydrogen ion + 2-Hydroxy-2,4-pentadienoate Water + 2-Hydroxy-2,4-pentadienoate <> 4-Hydroxy-2-oxopentanoate 2-Hydroxy-2,4-pentadienoate + Succinic acid <> 2-Hydroxy-6-ketononadienedicarboxylate + Water 2-Hydroxy-6-ketononatrienedioate + Water <> 2-Hydroxy-2,4-pentadienoate + Fumaric acid 2-Hydroxy-2,4-pentadienoate > 2-oxopent-4-enoate 4-Hydroxy-2-oxopentanoate > 2-Hydroxy-2,4-pentadienoate + Water 2-Hydroxy-6-ketononatrienedioate + Water > Hydrogen ion + Fumaric acid + 2-Hydroxy-2,4-pentadienoate + 2-Hydroxy-2,4-pentadienoate 2-oxopent-4-enoate + 2-Hydroxy-2,4-pentadienoate > Water + 4-hydroxy-2-oxopentanoate + 4-Hydroxy-2-oxopentanoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the cleavage of the C5-C6 bond of 2-hydroxy-6- oxononadienedioate and 2-hydroxy-6-oxononatrienedioate, a dienol ring fission product of the bacterial meta-cleavage pathway for degradation of phenylpropionic acid
- Gene Name:
- mhpC
- Uniprot ID:
- P77044
- Molecular weight:
- 32585
Reactions
| (2E,4Z)-2-hydroxy-6-oxonona-2,4-diene-1,9-dioate + H(2)O = (2E)-2-hydroxypenta-2,4-dienoate + succinate. |
| (2E,4Z,7E)-2-hydroxy-6-oxonona-2,4,7-triene-1,9-dioate + H(2)O = (2E)-2-hydroxypenta-2,4-dienoate + fumarate. |
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the conversion of 2-hydroxypentadienoic acid (enolic form of 2-oxopent-4-enoate) to 4-hydroxy-2-ketopentanoic acid
- Gene Name:
- mhpD
- Uniprot ID:
- P77608
- Molecular weight:
- 28890
Reactions
| 4-hydroxy-2-oxopentanoate = 2-oxopent-4-enoate + H(2)O. |