| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:21:24 -0600 |
|---|
| Update Date | 2015-06-03 17:19:04 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
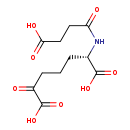
| Name: | N-Succinyl-2-amino-6-ketopimelate |
|---|
| Description | N-Succinyl-2-amino-6-ketopimelate is an intermediate in lysine biosynthesis. It is the fourth to last step in n the synthesis of lysine and is converted from tetrahydrodipicolinate via the enzyme tetrahydrodipicolinate N-succinyltransferase (EC 2.3.1.117). It is then converted to N-succinyl-L,L-2,6-diaminopimelate via the enzyme Succinyldiaminopimelate transferase (EC 2.6.1.17). |
|---|
| Structure | |
|---|
| Synonyms: | - (2S)-2-(3-carboxypropanamido)-6-oxoheptanedioate
- (2S)-2-(3-carboxypropanamido)-6-oxoheptanedioic acid
- (S)-2-(Succinylamino)-6-oxoheptanedioate
- (S)-2-(Succinylamino)-6-oxoheptanedioic acid
- N-succinyl-L-2-amino-6-oxoheptanedioate
- L-2-Succinylamino-6-oxoheptanedioate
- L-2-Succinylamino-6-oxoheptanedioic acid
- N-Succinyl-2-amino-6-ketopimelic acid
- N-Succinyl-2-amino-6-oxo-L-pimelate
- N-Succinyl-2-amino-6-oxo-L-pimelic acid
- N-Succinyl-2-L-amino-6-oxoheptanedioate
- N-Succinyl-2-L-amino-6-oxoheptanedioic acid
- N-Succinyl-epsilon-keto-L-aminopimelate
- N-Succinyl-epsilon-keto-L-aminopimelic acid
- N-Succinyl-L-2-amino-6-oxoheptanedioate
- N-Succinyl-L-2-amino-6-oxoheptanedioic acid
- N-Succinyl-L-2-amino-6-oxopimelate
- N-Succinyl-L-2-amino-6-oxopimelic acid
- SAKPA
- Succinyl-ε-keto-α-aminopimelate
- Succinyl-ε-keto-α-aminopimelic acid
- Succinyl-epsilon-keto-a-aminopimelate
- Succinyl-epsilon-keto-a-aminopimelic acid
- Succinyl-epsilon-keto-alpha-aminopimelate
- Succinyl-epsilon-keto-alpha-aminopimelic acid
- Succinyl-epsilon-keto-α-aminopimelate
- Succinyl-epsilon-keto-α-aminopimelic acid
|
|---|
| Chemical Formula: | C11H15NO8 |
|---|
| Weight: | Average: 289.2387
Monoisotopic: 289.079766461 |
|---|
| InChI Key: | SDVXSCSNVVZWDD-LURJTMIESA-N |
|---|
| InChI: | InChI=1S/C11H15NO8/c13-7(11(19)20)3-1-2-6(10(17)18)12-8(14)4-5-9(15)16/h6H,1-5H2,(H,12,14)(H,15,16)(H,17,18)(H,19,20)/t6-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2S)-2-(3-carboxypropanamido)-6-oxoheptanedioic acid |
|---|
| Traditional IUPAC Name: | (2S)-2-(3-carboxypropanamido)-6-oxoheptanedioic acid |
|---|
| SMILES: | OC(=O)CCC(=O)N[C@@H](CCCC(=O)C(O)=O)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-l-alpha-amino acids. These are n-acylated alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-l-alpha-amino acid
- Tricarboxylic acid or derivatives
- Alpha-keto acid
- Fatty amide
- Keto acid
- Fatty acyl
- N-acyl-amine
- Alpha-hydroxy ketone
- Carboxamide group
- Secondary carboxylic acid amide
- Ketone
- Carboxylic acid
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | -3 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | Water + Succinyl-CoA + Tetrahydrodipicolinate <> Coenzyme A + N-Succinyl-2-amino-6-ketopimelatealpha-Ketoglutarate + N-Succinyl-L,L-2,6-diaminopimelate <> L-Glutamate + N-Succinyl-2-amino-6-ketopimelateOxoglutaric acid + N-Succinyl-L,L-2,6-diaminopimelate <> L-Glutamate + N-Succinyl-2-amino-6-ketopimelateN-succinyl-L-2,6-diaminoheptanedioate + Oxoglutaric acid > N-Succinyl-2-amino-6-ketopimelate + L-GlutamateSuccinyl-CoA + (S)-2,3,4,5-tetrahydropyridine-2,6-dicarboxylate + Water > CoA + N-Succinyl-2-amino-6-ketopimelate(S)-2,3,4,5-tetrahydrodipicolinate + Succinyl-CoA + Water + Succinyl-CoA > Coenzyme A + N-Succinyl-2-amino-6-ketopimelateN-Succinyl-2-amino-6-ketopimelate + L-Glutamic acid + L-Glutamate > N-Succinyl-L,L-2,6-diaminopimelate + Oxoglutaric acidalpha-Ketoglutarate + N-Succinyl-L,L-2,6-diaminopimelate <> L-Glutamate + N-Succinyl-2-amino-6-ketopimelateWater + Succinyl-CoA + Tetrahydrodipicolinate <> Coenzyme A + N-Succinyl-2-amino-6-ketopimelate |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9880000000-0f0ff4f7e373c0b3162c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00du-9408600000-fd466a0230c24aa5db92 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fkc-0290000000-2cf1a6c8c873c44c176a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fdo-1970000000-e6da5433a70177935ddd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05i3-7900000000-a6e1749a519577b39b0b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-007c-0190000000-cfcf85c3db41c68abca2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-009g-1590000000-73abaab210b9727b9f16 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-5910000000-b01ea1042bed0446bc1c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0790000000-d2b9c6f92d7b00453793 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0abc-4930000000-f2f2e3269f8edd762cf8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-9700000000-787d11c10df4c3cb6d65 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-0590000000-2961efd3a4f8e021c893 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002g-0960000000-6adfe9927596ba357d75 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9500000000-70678263ddcfe89f4fb6 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|