Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
2,3-Diketo-L-gulonate (M2MDB000677)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 14:08:43 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-09-17 15:41:19 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2,3-Diketo-L-gulonate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 2,3-Diketo-L-gulonate is an intermediate in Ascorbate and aldarate metabolism. 2,3-Diketo-L-gulonate is produced from Dehydroascorbate and then converted to L-Xylonate via the enzyme Lyases (EC 4.1.1.-). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
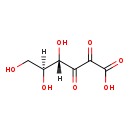
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H8O7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 192.1235 Monoisotopic: 192.02700261 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | GJQWCDSAOUMKSE-SCQFTWEKSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H8O7/c7-1-2(8)3(9)4(10)5(11)6(12)13/h2-3,7-9H,1H2,(H,12,13)/t2-,3?/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (4S,5S)-4,5,6-trihydroxy-2,3-dioxohexanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (4S,5S)-4,5,6-trihydroxy-2,3-dioxohexanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC[C@H](O)C(O)C(=O)C(=O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic oxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Sugar acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2,3-Diketo-L-gulonate + Hydrogen ion + NADH > 3-Dehydro-L-gulonate + NAD 3-Dehydro-L-gulonate + NAD <> 2,3-Diketo-L-gulonate + NADH + Hydrogen ion 3-Dehydro-L-gulonate + NADP <> 2,3-Diketo-L-gulonate + NADPH + Hydrogen ion dehydroascorbate (bicyclic form) > 2,3-Diketo-L-gulonate + Hydrogen ion 2,3-Diketo-L-gulonate > 2-carboxy-L-xylonolactone 3-Dehydro-L-gulonate + NAD < Hydrogen ion + 2,3-Diketo-L-gulonate + NADH 3-Dehydro-L-gulonate + NAD + NADP <> 2,3-Diketo-L-gulonate + NADH + NADPH + Hydrogen ion 2,3-Diketo-L-gulonate + NADH + Hydrogen ion + 2,3-Diketo-L-gulonate > 3-Dehydro-L-gulonate + NAD 2,3-Diketo-L-gulonate + Hydrogen ion + 2,3-Diketo-L-gulonate > Carbon dioxide + Xylulose 5-phosphate + Xylulose 5-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor
- Specific function:
- Catalyzes the reduction of 2,3-diketo-L-gulonate in the presence of NADH, to form 3-keto-L-gulonate
- Gene Name:
- dlgD
- Uniprot ID:
- P37672
- Molecular weight:
- 36572
Reactions
| 3-dehydro-L-gulonate + NAD(P)(+) = (4R,5S)-4,5,6-trihydroxy-2,3-dioxohexanoate + NAD(P)H. |
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the decarboxylation of 3-keto-L-gulonate-6-P into L-xylulose-5-P. May be involved in the utilization of 2,3- diketo-L-gulonate
- Gene Name:
- sgbH
- Uniprot ID:
- P37678
- Molecular weight:
- 23445
Reactions
| 3-dehydro-L-gulonate 6-phosphate = L-xylulose 5-phosphate + CO(2). |
Transporters
- General function:
- Carbohydrate transport and metabolism
- Specific function:
- Part of the tripartite ATP-independent periplasmic (TRAP) transport system yiaMNO involved in the uptake of 2,3- diketo-L-gulonate
- Gene Name:
- yiaM
- Uniprot ID:
- P37674
- Molecular weight:
- 17516
- General function:
- Carbohydrate transport and metabolism
- Specific function:
- Part of the tripartite ATP-independent periplasmic (TRAP) transport system yiaMNO involved in the uptake of 2,3- diketo-L-gulonate
- Gene Name:
- yiaN
- Uniprot ID:
- P37675
- Molecular weight:
- 45368