| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:08:14 -0600 |
|---|
| Update Date | 2015-06-03 15:54:51 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
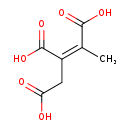
| Name: | Cis-2-Methylaconitate |
|---|
| Description | cis-2-Methylaconitate is produced due to the dehydration of 2-methylcitrate in 2-methylcitric acid cycle. The cycle is catalyzed by a cofactor-less (PrpD) enzyme or by an aconitase-like (AcnD) enzyme. (PMID: 17567742) |
|---|
| Structure | |
|---|
| Synonyms: | - (2Z)-but-2-ene-1,2,3-tricarboxylate
- (2Z)-but-2-ene-1,2,3-tricarboxylic acid
- (Z)-2-Butene-1,2,3-tricarboxylate
- (Z)-2-Butene-1,2,3-tricarboxylic acid
- (Z)-But-2-ene-1,2,3-tricarboxylate
- (Z)-But-2-ene-1,2,3-tricarboxylic acid
- 2-methyl-cis-aconitate
- 2-Methyl-cis-aconitate
- 2-Methyl-cis-aconitic acid
- 2-methylaconitate
- 2-Methylaconitic acid
- a-Methyl-cis-aconitate
- a-Methyl-cis-aconitic acid
- a-Methylaconitate
- a-Methylaconitic acid
- Alpha-Methyl-cis-aconitate
- Alpha-Methyl-cis-aconitic acid
- Alpha-Methylaconitate
- Alpha-Methylaconitic acid
- Cis-2-Butene-1,2,3-tricarboxylate
- Cis-2-Butene-1,2,3-tricarboxylic acid
- Cis-2-Methylaconitic acid
- α-Methyl-cis-aconitate
- α-Methyl-cis-aconitic acid
- α-Methylaconitate
- α-Methylaconitic acid
|
|---|
| Chemical Formula: | C7H8O6 |
|---|
| Weight: | Average: 188.1348
Monoisotopic: 188.032087988 |
|---|
| InChI Key: | NUZLRKBHOBPTQV-ARJAWSKDSA-N |
|---|
| InChI: | InChI=1S/C7H8O6/c1-3(6(10)11)4(7(12)13)2-5(8)9/h2H2,1H3,(H,8,9)(H,10,11)(H,12,13)/b4-3- |
|---|
| CAS number: | 6061-93-4 |
|---|
| IUPAC Name: | (1Z)-1-methylprop-1-ene-1,2,3-tricarboxylic acid |
|---|
| Traditional IUPAC Name: | α-methylaconitate |
|---|
| SMILES: | C\C(C(O)=O)=C(/CC(O)=O)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tricarboxylic acids and derivatives. These are carboxylic acids containing exactly three carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Tricarboxylic acids and derivatives |
|---|
| Direct Parent | Tricarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tricarboxylic acid or derivatives
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | -3 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | Cis-2-Methylaconitate + Water <> Methylisocitric acidMethylcitric acid + (2S,3S)-2-hydroxybutane-1,2,3-tricarboxylate <> Cis-2-Methylaconitate + WaterMethylcitric acid <> Cis-2-Methylaconitate + WaterMethylisocitric acid <> Cis-2-Methylaconitate + WaterMethylcitric acid > Water + Cis-2-Methylaconitate(2S,3R)-3-hydroxybutane-1,2,3-tricarboxylate > Cis-2-Methylaconitate + Water(2S,3S)-2-hydroxybutane-1,2,3-tricarboxylate > Cis-2-Methylaconitate + Water2-Methylcitric acid + Methylcitric acid > Water + Cis-2-MethylaconitateCis-2-Methylaconitate > Water + Methylisocitric acidMethylcitric acid + (2S,3S)-2-hydroxybutane-1,2,3-tricarboxylate <> Cis-2-Methylaconitate + WaterMethylcitric acid + (2S,3S)-2-hydroxybutane-1,2,3-tricarboxylate <> Cis-2-Methylaconitate + Water |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002g-6900000000-ffb445dd87ee618648ff | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0079-9388000000-0ff4121be9b872f0c1ab | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1900000000-202d4666a34f5b4f09f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00fs-5900000000-9c256387d352a7a6ebb2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002b-9500000000-027a2750edf1831b7088 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-1900000000-e925a682247370a2fa7f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mn-4900000000-ec6a931ab7e2e1653ea8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dj-9200000000-07d3197108036a4cb0ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002e-4900000000-fe6584e3d383728ee001 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9200000000-80caf61386c108fcd0b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9100000000-327d1a78edc99f8fe760 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fi0-0900000000-734e75ff99c6e837cdcb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-9600000000-065cce37ffa797ab854c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006t-9000000000-925f178b16f34b5de908 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Garvey, G. S., Rocco, C. J., Escalante-Semerena, J. C., Rayment, I. (2007). "The three-dimensional crystal structure of the PrpF protein of Shewanella oneidensis complexed with trans-aconitate: insights into its biological function." Protein Sci 16:1274-1284. Pubmed: 17567742
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: | Tabuchi, Takeshi; Uchiyama, Hiroo. Methylcitrate condensing and methylisocitrate cleaving enzymes. Evidence for the pathway of oxidation of propionyl-CoA to pyruvate via C7-tricarboxylic acids. Agricultural and Biological Chemistry (1975), 39(10), |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|