| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 13:58:28 -0600 |
|---|
| Update Date | 2015-09-13 12:56:12 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Bilirubin diglucuronide |
|---|
| Description | Bilirubin diglucuronide is a water soluble version of bilirubin. E. coli living in the mammalian gut can use this compound as a substrate for growth. Bilirubin glucuronides are water-soluble. |
|---|
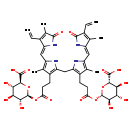
| Structure | |
|---|
| Synonyms: | - (2S,3S,4S,5R,6S)-6-[3-[2-[[3-[2-[(2S,3R,4S,5S,6S)-6-carboxy-3,4,5-trihydroxy-oxan-2-yl]oxycarbonylethyl]-5-[(E)-(3-ethenyl-4-methyl-5-oxo-pyrrol-2-ylidene)methyl]-4-methyl-1H-pyrrol-2-yl]methyl]-5-[(E)-(4-ethenyl-3-methyl-5-oxo-pyrrol-2-ylidene)methyl]-4-methyl-1H-pyrrol-3-yl]propanoyloxy]-3,4,5-trihydroxy-oxane-2-carboxylate
- (2S,3S,4S,5R,6S)-6-[3-[2-[[3-[2-[(2S,3R,4S,5S,6S)-6-carboxy-3,4,5-trihydroxy-oxan-2-yl]oxycarbonylethyl]-5-[(E)-(3-ethenyl-4-methyl-5-oxo-pyrrol-2-ylidene)methyl]-4-methyl-1H-pyrrol-2-yl]methyl]-5-[(E)-(4-ethenyl-3-methyl-5-oxo-pyrrol-2-ylidene)methyl]-4-methyl-1H-pyrrol-3-yl]propanoyloxy]-3,4,5-trihydroxy-oxane-2-carboxylic acid
- Bilirubin β-diglucuronide
- Bilirubin b-diglucuronide
- Bilirubin beta-diglucuronide
- Bilirubin β-diglucuronide
- Bilirubin-bisglucuronoside
- Bis(glucosyluronate)bilirubin
- Bis(glucosyluronic acid)bilirubin
|
|---|
| Chemical Formula: | C45H52N4O18 |
|---|
| Weight: | Average: 936.921
Monoisotopic: 936.32766085 |
|---|
| InChI Key: | SCJLWMXOOYZBTH-BTVQFETGSA-N |
|---|
| InChI: | InChI=1S/C45H52N4O18/c1-7-20-19(6)40(58)49-27(20)14-25-18(5)23(10-12-31(51)65-45-37(57)33(53)35(55)39(67-45)43(62)63)29(47-25)15-28-22(17(4)24(46-28)13-26-16(3)21(8-2)41(59)48-26)9-11-30(50)64-44-36(56)32(52)34(54)38(66-44)42(60)61/h7-8,13-14,32-39,44-47,52-57H,1-2,9-12,15H2,3-6H3,(H,48,59)(H,49,58)(H,60,61)(H,62,63)/b26-13-,27-14-/t32-,33-,34-,35-,36+,37+,38-,39-,44+,45+/m0/s1 |
|---|
| CAS number: | 17459-92-6 |
|---|
| IUPAC Name: | (2S,3S,4S,5R,6S)-6-{[3-(2-{[3-(3-{[(2S,3R,4S,5S,6S)-6-carboxy-3,4,5-trihydroxyoxan-2-yl]oxy}-3-oxopropyl)-5-{[(2E)-3-ethenyl-5-hydroxy-4-methyl-2H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-2-yl]methyl}-5-{[(2E)-4-ethenyl-5-hydroxy-3-methyl-2H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoyl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional IUPAC Name: | (2S,3S,4S,5R,6S)-6-{[3-(2-{[3-(3-{[(2S,3R,4S,5S,6S)-6-carboxy-3,4,5-trihydroxyoxan-2-yl]oxy}-3-oxopropyl)-5-{[(2E)-3-ethenyl-5-hydroxy-4-methylpyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-2-yl]methyl}-5-{[(2E)-4-ethenyl-5-hydroxy-3-methylpyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoyl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| SMILES: | [H]\C(C1=C(C)C(CCC(=O)O[C@]2([H])O[C@]([H])(C(O)=O)[C@@]([H])(O)[C@]([H])(O)[C@@]2([H])O)=C(CC2=C(CCC(=O)O[C@]3([H])O[C@]([H])(C(O)=O)[C@@]([H])(O)[C@]([H])(O)[C@@]3([H])O)C(C)=C(N2)C(\[H])=C2/N=C(O)C(C)=C2C=C)N1)=C1\N=C(O)C(C=C)=C1C |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Bilirubins |
|---|
| Direct Parent | Bilirubins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bilirubin skeleton
- O-glucuronide
- 1-o-glucuronide
- Tetracarboxylic acid or derivatives
- Glucuronic acid or derivatives
- Beta-hydroxy acid
- Fatty acid ester
- Monosaccharide
- Oxane
- Pyran
- Substituted pyrrole
- Fatty acyl
- Hydroxy acid
- Pyrroline
- Pyrrole
- Heteroaromatic compound
- Secondary alcohol
- Secondary carboxylic acid amide
- Carboxylic acid ester
- Lactam
- Carboxamide group
- Acetal
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Azacycle
- Polyol
- Organic oxide
- Alcohol
- Organopnictogen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | - Porphyrin and chlorophyll metabolism ec00860
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kf-0200030903-3f731d3255f730a58952 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016u-0300070902-a0595a946705461ddb24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0059-2200094431-2cedb95f9845511ac143 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052f-0200025912-c488140c352914da3532 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kf-2800020923-c9e3902c3b856732b595 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01r7-6900003740-e2aa55faca0003c1270f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014l-0010500539-05ec0afe18cb6d6a4983 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0230530967-386f3f3c90b3b8a6bb6c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udr-3490340563-80e35a547643c4a2b391 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0540-1720170916-65c2b0b7a27f92bc8cb7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0670-5350061975-523df2bd3cff46ef40a4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-5090120520-83d0638c5c3df842db5d | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| Links |
|---|
| External Links: | |
|---|