| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 13:53:37 -0600 |
|---|
| Update Date | 2015-09-17 15:41:12 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Queuine |
|---|
| Description | Queuine is a member of the chemical class known as Aminocyclitols and Derivatives. These are cyclitols with at least one hydroxyl group replace by an amino group. Queuine (Q) is a hypermodified base found in the first (or wobble) position of the anticodon of tRNAs specific for Asn, Asp, His, and Tyr, in most eukaryotes and prokaryotes. The nucleoside of queuine is queuosine. Queuine is not found in the tRNA of archaea; however, a related 7-deazaguanine derivative, the nucleoside of which is archaeosine, occurs in different tRNA position, the dihydrouridine loop, and in tRNAs with more specificities. (WikiPedia) |
|---|
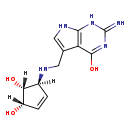
| Structure | |
|---|
| Synonyms: | - 2-Amino-5-((((1S,4S,5R)-4,5-dihydroxy-2-cyclopenten-1-yl)amino)methyl)-1,7-dihydro-4H-pyrrolo(2,3-D)pyrimidin-4-one
- 7-(3,4-trans-4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanine
- 7AMe7DAGua
- Base Q
- Queuine
|
|---|
| Chemical Formula: | C12H15N5O3 |
|---|
| Weight: | Average: 277.2792
Monoisotopic: 277.117489371 |
|---|
| InChI Key: | WYROLENTHWJFLR-BHNWBGBOSA-N |
|---|
| InChI: | InChI=1S/C12H15N5O3/c13-12-16-10-8(11(20)17-12)5(4-15-10)3-14-6-1-2-7(18)9(6)19/h1-2,4,6-7,9,14,18-19H,3H2,(H4,13,15,16,17,20)/t6-,7-,9+/m1/s1 |
|---|
| CAS number: | 72496-59-4 |
|---|
| IUPAC Name: | (1R,2S,5S)-5-[({4-hydroxy-2-imino-1H,2H,7H-pyrrolo[2,3-d]pyrimidin-5-yl}methyl)amino]cyclopent-3-ene-1,2-diol |
|---|
| Traditional IUPAC Name: | (1R,2S,5S)-5-[({4-hydroxy-2-imino-1H,7H-pyrrolo[2,3-d]pyrimidin-5-yl}methyl)amino]cyclopent-3-ene-1,2-diol |
|---|
| SMILES: | O[C@@H]1C=C[C@@H](NCC2=CNC3=C2C(=O)NC(=N)N3)[C@@H]1O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrrolo[2,3-d]pyrimidines. These are aromatic heteropolycyclic compounds containing a pyrrolo[2,3-d]pyrimidine ring system, which is an pyrrolopyrimidine isomers having the 3 ring nitrogen atoms at the 1-, 5-, and 7-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrrolopyrimidines |
|---|
| Sub Class | Pyrrolo[2,3-d]pyrimidines |
|---|
| Direct Parent | Pyrrolo[2,3-d]pyrimidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrrolo[2,3-d]pyrimidine
- Hydroxypyrimidine
- Aralkylamine
- Pyrimidine
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- 1,2-diol
- Secondary alcohol
- Secondary aliphatic amine
- Azacycle
- Secondary amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|