| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 13:01:40 -0600 |
|---|
| Update Date | 2015-06-03 15:53:36 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
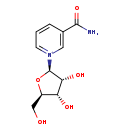
| Name: | Nicotinamide riboside |
|---|
| Description | Nicotinamide riboside is involved in nicotinate and nicotinamide metabolism. It is a reducible moiety of the coenzyme NAD+. Nicotinamide riboside kinase has an essential role for phosphorylation of nicotinamide riboside (PMID 15137942). |
|---|
| Structure | |
|---|
| Synonyms: | - 1-(β-D ribofuranosyl)nicotinamide
- 1-(b-D Ribofuranosyl)nicotinamide
- 1-(b-D-Ribofuranosyl)-nicotinamide
- 1-(b-D-Ribofuranosyl)nicotinamide
- 1-(beta-D Ribofuranosyl)nicotinamide
- 1-(beta-D-ribofuranosyl)-nicotinamide
- 1-(beta-D-Ribofuranosyl)nicotinamide
- 1-(β-D Ribofuranosyl)nicotinamide
- 1-(β-D-Ribofuranosyl)-nicotinamide
- 1-(β-D-Ribofuranosyl)nicotinamide
- 1-b-D-Ribosyl-3-Pyridinecarboxamide
- 1-b-delta-Ribosyl-3-pyridinecarboxamide
- 1-b-δ-Ribosyl-3-pyridinecarboxamide
- 1-beta-D-Ribosyl-3-Pyridinecarboxamide
- 1-beta-delta-Ribosyl-3-Pyridinecarboxamide
- 1-β-D-Ribosyl-3-pyridinecarboxamide
- 1-β-δ-Ribosyl-3-pyridinecarboxamide
- 3-(Aminocarbonyl)-1-b-D-ribofuranosyl-pyridinium
- 3-(Aminocarbonyl)-1-b-delta-ribofuranosyl-pyridinium
- 3-(Aminocarbonyl)-1-b-δ-ribofuranosyl-pyridinium
- 3-(Aminocarbonyl)-1-beta-D-ribofuranosyl-Pyridinium
- 3-(Aminocarbonyl)-1-beta-delta-ribofuranosyl-Pyridinium
- 3-(Aminocarbonyl)-1-β-D-ribofuranosyl-pyridinium
- 3-(Aminocarbonyl)-1-β-δ-ribofuranosyl-pyridinium
- N-ribosyl-nicotinamide
- N-Ribosyl-nicotinamide
- N-Ribosylnicotinamide
- Nicotinamide ribonucleoside
- Nicotinamide ribose
- Nicotinamide riboside
- Nicotinamide-b-riboside
- Nicotinamide-beta-riboside
- Nicotinamide-β-riboside
- Ribosylnicotinamide
|
|---|
| Chemical Formula: | C11H15N2O5 |
|---|
| Weight: | Average: 255.2472
Monoisotopic: 255.0980966 |
|---|
| InChI Key: | JLEBZPBDRKPWTD-TURQNECASA-O |
|---|
| InChI: | InChI=1S/C11H14N2O5/c12-10(17)6-2-1-3-13(4-6)11-9(16)8(15)7(5-14)18-11/h1-4,7-9,11,14-16H,5H2,(H-,12,17)/p+1/t7-,8-,9-,11-/m1/s1 |
|---|
| CAS number: | 1341-23-7 |
|---|
| IUPAC Name: | 3-carbamoyl-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1lambda5-pyridin-1-ylium |
|---|
| Traditional IUPAC Name: | 3-carbamoyl-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1lambda5-pyridin-1-ylium |
|---|
| SMILES: | NC(=O)C1=C[N+](=CC=C1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glycosylamines. Glycosylamines are compounds consisting of an amine with a beta-N-glycosidic bond to a carbohydrate, thus forming a cyclic hemiaminal ether bond (alpha-amino ether). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Glycosylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-glycosyl compound
- Pentose monosaccharide
- Nicotinamide
- Pyridine carboxylic acid or derivatives
- Monosaccharide
- Pyridine
- Pyridinium
- Vinylogous amide
- Heteroaromatic compound
- Tetrahydrofuran
- Secondary alcohol
- Carboxamide group
- Primary carboxylic acid amide
- Organoheterocyclic compound
- Oxacycle
- Azacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Primary alcohol
- Organic oxide
- Organonitrogen compound
- Organopnictogen compound
- Alcohol
- Organic nitrogen compound
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | - Nicotinate and nicotinamide metabolism ec00760
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-070f-9530000000-71d9e02ee640bb800185 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0a6r-9825400000-99de0fc632f408e32a12 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 0V, positive | splash10-0a4i-0190000000-51bf764225f08b566d36 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 1V, positive | splash10-0a4i-0290000000-ffbe280293f874204044 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, positive | splash10-05fr-0970000000-1714e669425e34d3754a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, positive | splash10-00di-0920000000-77ef12950b3152d752c8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 5V, positive | splash10-00di-0900000000-b14d15851532222ba74a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 6V, positive | splash10-00di-0900000000-277d95be8f0485eb3ce5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 7V, positive | splash10-00di-0900000000-354c1f3978635e5109bb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 8V, positive | splash10-00di-0900000000-ea5e376f71c6414964bc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 10V, positive | splash10-00di-0900000000-6b289ae1c5f53e92f774 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 11V, positive | splash10-00di-1900000000-6ad92fa16e2cca17f959 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 12V, positive | splash10-00di-1900000000-cee8757ed6b406ccf044 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 15V, positive | splash10-00di-2900000000-78f21d967892a0d9ab85 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 19V, positive | splash10-00di-3900000000-cd1f66bfc8ded7bd098e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 22V, positive | splash10-00e9-8900000000-5a6bc8100043575c6a58 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 28V, positive | splash10-00ai-9200000000-aedd3c5bf179b666a6ae | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 33V, positive | splash10-003r-9000000000-22053c801abdfaf5f562 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 41V, positive | splash10-0fai-9000000000-b6b4b7e819e08c863a9a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 17V, positive | splash10-00di-0900000000-3364e345334aa7f5a742 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 17V, positive | splash10-004i-9000000000-3bf262f96423e85a1756 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-e25d1beb47cb1fe9e4cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-3090000000-03687e9ef697eda1d6c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052g-9300000000-2755bde4df843d2227f2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-809be1146b2a2c8d96a7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1390000000-2fc72cf0619f182e044f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k96-9300000000-4d5b96b192f3484b1b8a | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C: Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007 May 4;129(3):473-84. Pubmed: 17482543
- Bieganowski, P., Brenner, C. (2004). "Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans." Cell 117:495-502. Pubmed: 15137942
- Bogan KL, Brenner C: Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115-30. doi: 10.1146/annurev.nutr.28.061807.155443. Pubmed: 18429699
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J: The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012 Jun 6;15(6):838-47. doi: 10.1016/j.cmet.2012.04.022. Pubmed: 22682224
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S: Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci. 2004 Jan;61(1):19-34. Pubmed: 14704851
- Pankiewicz KW, Watanabe KA, Lesiak-Watanabe K, Goldstein BM, Jayaram HN: The chemistry of nicotinamide adenine dinucleotide (NAD) analogues containing C-nucleosides related to nicotinamide riboside. Curr Med Chem. 2002 Apr;9(7):733-41. Pubmed: 11966436
- Schalk-Hihi C, Zhang YZ, Markham GD: The conformation of NADH bound to inosine 5'-monophosphate dehydrogenase determined by transferred nuclear Overhauser effect spectroscopy. Biochemistry. 1998 May 19;37(20):7608-16. Pubmed: 9585576
- Wall KA, Klis M, Kornet J, Coyle D, Ame JC, Jacobson MK, Slama JT: Inhibition of the intrinsic NAD+ glycohydrolase activity of CD38 by carbocyclic NAD analogues. Biochem J. 1998 Nov 1;335 ( Pt 3):631-6. Pubmed: 9794804
|
|---|
| Synthesis Reference: | Franchetti, Palmarisa; Pasqualini, Michela; Petrelli, Riccardo; Ricciutelli, Massimo; Vita, Patrizia; Cappellacci, Loredana. Stereoselective synthesis of nicotinamide b-riboside and nucleoside analogs. Bioorganic & Medicinal Chemistry Letters (2004), 1 |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|